Measurement of changes in uterine and fibroid volume during treatment of heavy menstrual bleeding (HMB)
- PMID: 37304815
- PMCID: PMC10247393
- DOI: 10.1093/hropen/hoad021
Measurement of changes in uterine and fibroid volume during treatment of heavy menstrual bleeding (HMB)
Abstract
Study question: Does application of an unbiased method for analysis of magnetic resonance (MR) images reveal any effect on uterine or fibroid volume from treatment of heavy menstrual bleeding (HMB) with three 12-week courses of the selective progesterone receptor modulator ulipristal acetate (SPRM-UPA)?
Summary answer: Application of an unbiased method for analysis of MR images showed that treatment of HMB with SPRM-UPA was not associated with a significant reduction in the volume of the uterus or in the volume of uterine fibroids.
What is known already: SPRM-UPA shows therapeutic efficacy for treating HMB. However, the mechanism of action (MoA) is not well understood and there have been mixed reports, using potentially biased methodology, regarding whether SPRM-UPA has an effect on the volume of the uterus and fibroids.
Study design size duration: In a prospective clinical study (with no comparator), 19 women with HMB were treated over a period of 12 months with SPRM-UPA and uterine and fibroid size were assessed with high resolution structural MRI and stereology.
Participants/materials setting methods: A cohort of 19 women aged 38-52 years (8 with and 11 without fibroids) were treated with three 12-week courses of 5 mg SPRM-UPA given daily, with four weeks off medication in-between treatment courses. Unbiased estimates of the volume of uterus and total volume of fibroids were obtained at baseline, and after 6 and 12 months of treatment, by using the Cavalieri method of modern design-based stereology in combination with magnetic resonance imaging (MRI).
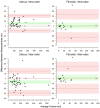
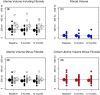
Main results and the role of chance: Bland-Altman plots showed good intra-rater repeatability and good inter-rater reproducibility for measurement of the volume of both fibroids and the uterus. For the total patient cohort, two-way ANOVA did not show a significant reduction in the volume of the uterus after two or three treatment courses of SPRM-UPA (P = 0.51), which was also the case when the groups of women with and without fibroids were considered separately (P = 0.63). One-way ANOVA did not show a significant reduction in total fibroid volume in the eight patients with fibroids (P = 0.17).
Limitations reasons for caution: The study has been performed in a relatively small cohort of women and simulations that have subsequently been performed using the acquired data have shown that for three time points and a group size of up to 50, with alpha (Type I Error) and beta (Type II Error) set to 95% significance and 80% power, respectively, at least 35 patients would need to be recruited in order for the null hypothesis (that there is no significant reduction in total fibroid volume) to be potentially rejected.
Wider implications of the findings: The imaging protocol that we have developed represents a generic paradigm for measuring the volume of the uterus and uterine fibroids that can be readily incorporated in future studies of medical treatments of HMB. In the present study, SPRM-UPA failed to produce a significant reduction in the volume of the uterus or the total volume of fibroids (which were present in approximately half of the patients) after either two or three 12-week courses of treatment. This finding represents a new insight in respect of the management of HMB using treatment strategies that target hormone-dependence.
Study funding/competing interests: The UPA Versus Conventional Management of HMB (UCON) trial was funded by the EME Programme (Medical Research Council (MRC) and National Institutes of Health Research (NIHR)) (12/206/52). The views expressed in this publication are those of the authors and not necessarily those of the Medical Research Council, National Institute for Health Research, or Department of Health and Social Care.Medical Research Council (MRC) Centre grants to the Centre for Reproductive Health (CRH) (G1002033 and MR/N022556/1) are also gratefully acknowledged. H.C. has clinical research support for laboratory consumables and staff from Bayer AG and provides consultancy advice (All paid to Institution) for Bayer AG, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd, AbbVie Inc., and Myovant Sciences GmbH. H.C. has received royalties from UpToDate for an article on abnormal uterine bleeding. L.W. has received grant funding from Roche Diagnostics (Paid to Institution). All other authors have no conflicts to declare.
Trial registration number: The study reported here is an embedded mechanism of action study (no comparator) within the UCON clinical trial (registration ISRCTN: 20426843).
Keywords: adenomyosis; fibroids; imaging; leiomyoma; progesterone; uterus.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
H.C. has clinical research support for laboratory consumables and staff from Bayer AG (Paid to Institution) and provides consultancy advice (Paid to Institution) for Bayer AG, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd, AbbVie Inc., and Myovant Sciences GmbH. H.C. has received royalties from UpToDate for an article on abnormal uterine bleeding. L.W. has received grant funding from Roche Diagnostics (Paid to Institution). All other authors have no conflicts to declare.
Figures
Similar articles
-
Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials.Hum Reprod. 2019 Apr 1;34(4):623-634. doi: 10.1093/humrep/dez007. Hum Reprod. 2019. PMID: 30865281 Free PMC article. Clinical Trial.
-
Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium.Hum Reprod. 2017 Mar 1;32(3):531-543. doi: 10.1093/humrep/dew359. Hum Reprod. 2017. PMID: 28130434 Free PMC article.
-
Individualized vaginal bleeding experience of women with uterine fibroids in the PEARL I randomized controlled trial comparing the effects of ulipristal acetate or placebo.Hum Reprod. 2014 Mar;29(3):480-9. doi: 10.1093/humrep/det467. Epub 2014 Jan 23. Hum Reprod. 2014. PMID: 24457604 Clinical Trial.
-
Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding: the UCON randomised controlled trial and mechanism of action study.Southampton (UK): National Institute for Health and Care Research; 2023 Oct. Southampton (UK): National Institute for Health and Care Research; 2023 Oct. PMID: 37931048 Free Books & Documents. Review.
-
[Selective progesterone receptor modulator (ulipristal acetate--a new option in the pharmacological treatment of uterine fibroids in women].Ginekol Pol. 2013 Mar;84(3):219-22. doi: 10.17772/gp/1567. Ginekol Pol. 2013. PMID: 23700851 Review. Polish.
References
-
- Baggio S, Pomini P, Galeone F, Presti F, Santi L, Raffaelli R, Franchi M.. Influence of ulipristal acetate therapy on uterine fibroid-related symptoms and on uterine and fibroid volumes and vascularity indices assessed by ultrasound. J Ultrasound Med 2018;37:2215–2223. - PubMed
-
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM.. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107. - PubMed
-
- Becker E Jr, Lev-Toaff AS, Kaufman EP, Halpern EJ, Edelweiss MI, Kurtz AB.. The added value of transvaginal sonohysterography over transvaginal sonography alone in women with known or suspected leiomyoma. J Ultrasound Med 2002;21:237–247. - PubMed
-
- Bulun SE. Uterine fibroids. N Engl J Med 2013;369:1344–1355. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
