MFAP2 enhances cisplatin resistance in gastric cancer cells by regulating autophagy
- PMID: 37304872
- PMCID: PMC10257393
- DOI: 10.7717/peerj.15441
MFAP2 enhances cisplatin resistance in gastric cancer cells by regulating autophagy
Abstract
Background: Cisplatin (CDDP) is of importance in cancer treatment and widely used in advanced gastric cancer (GC). However, its clinical usage is limited due to its resistance, and the regulatory mechanism of CDDP resistance in GC has not yet been fully elucidated. In this study, we first conducted a comprehensive study to investigate the role of MFAP2 through bioinformatics analysis.
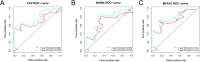
Methods: The Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) databases were applied to downloadgene expression data and clinicopathologic data, and the differentially expressed genes (DEGs) were further analyzed. Then, Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and survival analysis were conducted. Furthermore, according to the clinicopathological characteristics of TCGA, clinical correlation analysis was conducted, and a receiver operating characteristic curve (ROC) was plotted.
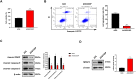
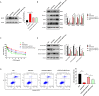
Results: We revealed that FAP, INHBA and MFAP2 were good diagnostic factors of GC. However, the mechanism of MFAP2 in GC remains elusive, especially in the aspect of chemotherapy resistance. We developed the CDDP-resistant cell line, and found that MFAP2 was upregulated in CDDP-resistant cells, and MFAP2-knockdown improved CDDP sensitivity. Finally, we found that MFAP2 enhanced CDDP resistance by inducing autophagy in drug-resistant cell lines.
Conclusions: The above results suggested that MFAP2 could affect the chemotherapy resistance by altering the level of autophagy in GC patients as a potential therapeutic target.
Keywords: Autophagy; CDDP-resistance; Gastric cancer; MFAP2.
©2023 Li et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures






Similar articles
-
Circular RNA MCTP2 inhibits cisplatin resistance in gastric cancer by miR-99a-5p-mediated induction of MTMR3 expression.J Exp Clin Cancer Res. 2020 Nov 17;39(1):246. doi: 10.1186/s13046-020-01758-w. J Exp Clin Cancer Res. 2020. PMID: 33198772 Free PMC article.
-
Functional and transcriptomic characterization of cisplatin-resistant AGS and MKN-28 gastric cancer cell lines.PLoS One. 2020 Jan 28;15(1):e0228331. doi: 10.1371/journal.pone.0228331. eCollection 2020. PLoS One. 2020. PMID: 31990955 Free PMC article.
-
KLF5-mediated aquaporin 3 activated autophagy to facilitate cisplatin resistance of gastric cancer.Immunopharmacol Immunotoxicol. 2023 Apr;45(2):140-152. doi: 10.1080/08923973.2022.2122498. Epub 2022 Sep 20. Immunopharmacol Immunotoxicol. 2023. PMID: 36083020
-
Molecular Mechanism of Resistance to Chemotherapy in Gastric Cancers, the Role of Autophagy.Curr Drug Targets. 2020;21(7):713-721. doi: 10.2174/1389450120666191127113854. Curr Drug Targets. 2020. PMID: 31775598 Review.
-
MicroRNAs as the critical regulators of cisplatin resistance in gastric tumor cells.Genes Environ. 2021 Jun 7;43(1):21. doi: 10.1186/s41021-021-00192-4. Genes Environ. 2021. PMID: 34099061 Free PMC article. Review.
Cited by
-
FOXA1-mediated transcription of MFAP2 facilitates cell growth, metastasis and cisplatin resistance in uterine corpus endometrial carcinoma.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 28. doi: 10.1007/s00210-025-04041-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40153018
-
Microfibrillar-associated protein-2 facilitates aggressive progression of oral squamous cell carcinoma cells through Kelch-like E3 ubiquitin ligase-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling pathway.Cytojournal. 2025 Jun 14;22:61. doi: 10.25259/Cytojournal_53_2025. eCollection 2025. Cytojournal. 2025. PMID: 40708830 Free PMC article.
-
Reversal of chemotherapy resistance in gastric cancer with traditional Chinese medicine as sensitizer: potential mechanism of action.Front Oncol. 2025 Feb 20;15:1524182. doi: 10.3389/fonc.2025.1524182. eCollection 2025. Front Oncol. 2025. PMID: 40052129 Free PMC article. Review.
-
PDLIM1 Inhibits Chemoresistance by Blocking DNA Damage Repair in Gastric Cancer.Recent Pat Anticancer Drug Discov. 2025;20(2):260-273. doi: 10.2174/0115748928307544240502064448. Recent Pat Anticancer Drug Discov. 2025. PMID: 38779728
References
-
- Hwang JR, Kim WY, Cho YJ, Ryu JY, Choi JJ, Jeong SY, Kim MS, Kim JH, Paik ES, Lee YY, Han HD, Lee JW. Chloroquine reverses chemoresistance via upregulation of p21(WAF1/CIP1) and autophagy inhibition in ovarian cancer. Cell Death & Disease. 2020;11:1034. doi: 10.1038/s41419-020-03242-x. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

