Zeolite Supported Pt for Depolymerization of Polyethylene by Induction Heating
- PMID: 37304911
- PMCID: PMC10251740
- DOI: 10.1021/acs.iecr.2c04568
Zeolite Supported Pt for Depolymerization of Polyethylene by Induction Heating
Abstract
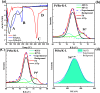
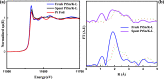
We demonstrate that for polyethylene depolymerization with induction heating (IH), using a bifunctional (Pt- or Pt-Sn-containing zeolite) hydrocracking catalyst, we can obtain high hydrocarbon product yields (up to 95 wt % in 2 h) at a relatively low surface temperature (375 °C) and with a tunable product distribution ranging from light gas products to gasoline- to diesel-range hydrocarbons. Four zeolite types [MFI, LTL, CHA(SSZ-13), and TON] were chosen as the supports due to their varying pore sizes and structures. These depolymerization results are obtained at atmospheric pressure and without the use of H2 and result in an alkane/alkene mixture with virtually no methane, aromatics, or coke formation. We also demonstrate how IH helps overcome diffusional resistances associated with conventional thermal heating and thereby shortens reaction times.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Catalytic Depolymerization of Waste Polyolefins by Induction Heating: Selective Alkane/Alkene Production.Ind Eng Chem Res. 2021 Oct 27;60(42):15141-15150. doi: 10.1021/acs.iecr.1c02674. Epub 2021 Oct 14. Ind Eng Chem Res. 2021. PMID: 34720395 Free PMC article.
-
Effects of Zn Addition into ZSM-5 Zeolite on Dehydrocyclization-Cracking of Soybean Oil Using Hierarchical Zeolite-Al2O3 Composite-Supported Pt/NiMo Sulfided Catalysts.ACS Omega. 2021 Feb 18;6(8):5509-5517. doi: 10.1021/acsomega.0c05855. eCollection 2021 Mar 2. ACS Omega. 2021. PMID: 33681592 Free PMC article.
-
Controlling liquid hydrocarbon composition in valorization of plastic waste via tuning zeolite framework and SiO2/Al2O3 ratio.J Environ Manage. 2021 Nov 1;297:113288. doi: 10.1016/j.jenvman.2021.113288. Epub 2021 Jul 20. J Environ Manage. 2021. PMID: 34298345
-
Fischer-Tropsch catalysts for the production of hydrocarbon fuels with high selectivity.ChemSusChem. 2014 May;7(5):1251-64. doi: 10.1002/cssc.201300797. Epub 2013 Dec 11. ChemSusChem. 2014. PMID: 24339240 Review.
-
New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels.Chem Soc Rev. 2019 Jun 17;48(12):3193-3228. doi: 10.1039/c8cs00502h. Chem Soc Rev. 2019. PMID: 31106785 Review.
Cited by
-
Magnetically Induced Catalysis: Definition, Advances, and Potential.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202424151. doi: 10.1002/anie.202424151. Epub 2025 Apr 18. Angew Chem Int Ed Engl. 2025. PMID: 40249118 Free PMC article.
-
Recent Progress in Polyolefin Plastic: Polyethylene and Polypropylene Transformation and Depolymerization Techniques.Molecules. 2024 Dec 29;30(1):87. doi: 10.3390/molecules30010087. Molecules. 2024. PMID: 39795145 Free PMC article. Review.
-
Predicting the effect of framework and hydrocarbon structure on the zeolite-catalyzed beta-scission.Catal Sci Technol. 2024 Oct 15;14(24):7020-7036. doi: 10.1039/d4cy00973h. eCollection 2024 Dec 9. Catal Sci Technol. 2024. PMID: 39421599 Free PMC article.
References
-
- Industry Agenda: The New Plastics Economy Rethinking the Future of Plastics; The World Economic Forum: Geneva, Switzerland, 2016; p 36.
-
- Rorrer J. E.; Troyano-Valls C.; Beckham G. T.; Román-Leshkov Y. Hydrogenolysis of polypropylene and mixed polyolefin plastic waste over Ru/C to produce liquid alkanes. ACS Sustainable Chem. Eng. 2021, 9, 11661–11666. 10.1021/acssuschemeng.1c03786. - DOI
-
- Britt P. F.; Coates G. W.; Winey K. I.. U.S. Department of Energy Report of the Basic Energy Sciences Roundtable on Chemical Upcycling of Polymers, 2019.
LinkOut - more resources
Full Text Sources
