Optimization of the Imaged cIEF Method for Monitoring the Charge Heterogeneity of Antibody-Maytansine Conjugate
- PMID: 37305029
- PMCID: PMC10256444
- DOI: 10.1155/2023/8150143
Optimization of the Imaged cIEF Method for Monitoring the Charge Heterogeneity of Antibody-Maytansine Conjugate
Abstract
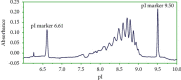
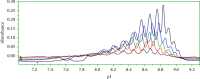
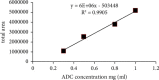
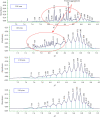
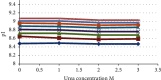
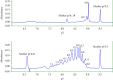
The aim of this study was to develop a whole-column imaging-detection capillary isoelectric focusing (icIEF) method for the analytical characterization of charge heterogeneity of a novel humanized anti-EphA2 antibody conjugated to a maytansine derivative. In addition to focusing time, sample composition was optimized: pH range, percent of carrier ampholytes, conjugated antibody concentration, and urea concentration. A good separation of charge isoforms was obtained with 4% carrier ampholytes of a large (3-10) and narrow pH range (8-10.5) (1 : 1 ratio), conjugated antibody concentration (0.3-1 mg/ml) with a good linearity (R2: 0.9905), 2 M of urea concentration, and 12 minute for focusing. The optimized icIEF method demonstrated a good interday repeatability with RSD values: <1% (pI), <8% (% peak area), and 7% (total peak areas). The optimized icIEF was useful as an analytical characterization tool to assess the charged isoform profile of a discovery batch of the studied maytansinoid-antibody conjugate in comparison to its naked antibody. It exhibited a large pI range (7.5-9.0), while its naked antibody showed a narrow pI range (8.9-9.0). In the discovery batch of maytansinoid-antibody conjugate, 2% of charge isoforms had the same pI as the pI of naked antibody isoforms.
Copyright © 2023 Ayat Abbood.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

