Optimization of Chitosan-Decorated Solid Lipid Nanoparticles for Improved Flurbiprofen Transdermal Delivery
- PMID: 37305303
- PMCID: PMC10249022
- DOI: 10.1021/acsomega.2c08135
Optimization of Chitosan-Decorated Solid Lipid Nanoparticles for Improved Flurbiprofen Transdermal Delivery
Abstract
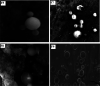
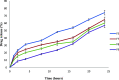
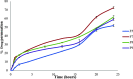
Transdermal delivery is a potential alternative route to oral administration for drugs associated with stomach discomfort, such as flurbiprofen, a widely nonsteroidal anti-inflammatory drug (NSAID). This study aimed to design solid lipid nanoparticle (SLN) transdermal formulations of flurbiprofen. Chitosan-coated SLNs were prepared by the solvent emulsification method, and their properties and permeation profiles across the excised rat skin were characterized. The particle size of uncoated SLNs was at 695 ± 4.65 nm, which increased to 714 ± 6.13, 847 ± 5.38, and 900 ± 8.65 nm upon coating with 0.05, 0.10, and 0.20% of chitosan, respectively. The drug association efficiency was improved when a higher concentration of chitosan was employed over SLN droplets that endowed a higher affinity of flurbiprofen with chitosan. The drug release was significantly retarded as compared to the uncoated entities and followed non-Fickian anomalous diffusion that was depicted by "n" values of >0.5 and <1. Also, the total permeation of chitosan-coated SLNs (F7-F9) was significantly higher than that of the noncoated formulation (F5). Overall, this study has successfully designed a suitable carrier system of chitosan-coated SLNs that provide insight into the current conventional therapeutic approaches and suggest new directions for the advancements in transdermal drug delivery systems for improved permeation of flurbiprofen.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Repaglinide-Solid Lipid Nanoparticles in Chitosan Patches for Transdermal Application: Box-Behnken Design, Characterization, and In Vivo Evaluation.Int J Nanomedicine. 2024 Jan 10;19:209-230. doi: 10.2147/IJN.S438564. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38223883 Free PMC article.
-
Solid lipid nanoparticles for transdermal delivery of avanafil: optimization, formulation, in-vitro and ex-vivo studies.J Liposome Res. 2016 Dec;26(4):288-96. doi: 10.3109/08982104.2015.1117490. Epub 2016 Jan 19. J Liposome Res. 2016. PMID: 26784833
-
Physicochemical Characterization of Chitosan-Decorated Finasteride Solid Lipid Nanoparticles for Skin Drug Delivery.Biomed Res Int. 2022 Aug 6;2022:7792180. doi: 10.1155/2022/7792180. eCollection 2022. Biomed Res Int. 2022. PMID: 35971450 Free PMC article.
-
Tacrolimus-Loaded Solid Lipid Nanoparticle Gel: Formulation Development and In Vitro Assessment for Topical Applications.Gels. 2022 Feb 18;8(2):129. doi: 10.3390/gels8020129. Gels. 2022. PMID: 35200510 Free PMC article.
-
Do the chitosan nanoparticles really augment the drugs' transdermal fluxes: ending the debate using meta-analysis.Expert Opin Drug Deliv. 2024 Feb;21(2):325-335. doi: 10.1080/17425247.2024.2317935. Epub 2024 Mar 1. Expert Opin Drug Deliv. 2024. PMID: 38340063 Review.
Cited by
-
Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics.Gels. 2025 May 13;11(5):358. doi: 10.3390/gels11050358. Gels. 2025. PMID: 40422378 Free PMC article. Review.
-
Development of propranolol loaded SLN for transdermal delivery: in-vitro characterization and skin deposition studies.Ther Deliv. 2025 Mar;16(3):205-215. doi: 10.1080/20415990.2025.2458451. Epub 2025 Jan 28. Ther Deliv. 2025. PMID: 39874079
-
The Science of Solid Lipid Nanoparticles: From Fundamentals to Applications.Cureus. 2024 Sep 6;16(9):e68807. doi: 10.7759/cureus.68807. eCollection 2024 Sep. Cureus. 2024. PMID: 39376878 Free PMC article. Review.
-
5-Fluorouracil-Loaded Hyaluronic Acid-Coated Niosomal Vesicles: Fabrication and Ex Vivo Evaluation for Skin Drug Delivery.ACS Omega. 2023 Nov 27;8(48):45405-45413. doi: 10.1021/acsomega.3c04457. eCollection 2023 Dec 5. ACS Omega. 2023. PMID: 38075815 Free PMC article.
-
Fabrication and evaluation of solidified nanoemulsion designs for systemic delivery of atorvastatin through the lung.Sci Rep. 2025 Jul 1;15(1):20884. doi: 10.1038/s41598-025-05646-1. Sci Rep. 2025. PMID: 40596164 Free PMC article.
References
-
- Chaturvedi S.; Garg A. An Insight of Techniques for the Assessment of Permeation Flux across the Skin for Optimization of Topical and Transdermal Drug Deliveryery Systems. J. Drug Delivery Sci. Technol. 2021, 62, 10235510.1016/j.jddst.2021.102355. - DOI
-
- Long L.-y.; Zhang J.; Yang Z.; Guo Y.; Hu X.; Wang Y. Transdermal Delivery of Peptide and Protein Drugs: Strategies, Advantages and Disadvantages. J. Drug Delivery Sci. Technol. 2020, 60, 10200710.1016/j.jddst.2020.102007. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

