Non-typeable Haemophilus influenzae major outer membrane protein P5 contributes to bacterial membrane stability, and affects the membrane protein composition crucial for interactions with the human host
- PMID: 37305414
- PMCID: PMC10250671
- DOI: 10.3389/fcimb.2023.1085908
Non-typeable Haemophilus influenzae major outer membrane protein P5 contributes to bacterial membrane stability, and affects the membrane protein composition crucial for interactions with the human host
Abstract
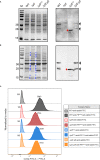
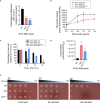
Non-typeable Haemophilus influenzae (NTHi) is a Gram-negative human pathogen that causes a wide range of airway diseases. NTHi has a plethora of mechanisms to colonize while evading the host immune system for the establishment of infection. We previously showed that the outer membrane protein P5 contributes to bacterial serum resistance by the recruitment of complement regulators. Here, we report a novel role of P5 in maintaining bacterial outer membrane (OM) integrity and protein composition important for NTHi-host interactions. In silico analysis revealed a peptidoglycan-binding motif at the periplasmic C-terminal domain (CTD) of P5. In a peptidoglycan-binding assay, the CTD of P5 (P5CTD) formed a complex with peptidoglycan. Protein profiling analysis revealed that deletion of CTD or the entire P5 changed the membrane protein composition of the strains NTHi 3655Δp5CTD and NTHi 3655Δp5, respectively. Relative abundance of several membrane-associated virulence factors that are crucial for adherence to the airway mucosa, and serum resistance were altered. This was also supported by similar attenuated pathogenic phenotypes observed in both NTHi 3655Δp5 CTD and NTHi 3655Δp5. We found (i) a decreased adherence to airway epithelial cells and fibronectin, (ii) increased complement-mediated killing, and (iii) increased sensitivity to the β-lactam antibiotics in both mutants compared to NTHi 3655 wild-type. These mutants were also more sensitive to lysis at hyperosmotic conditions and hypervesiculated compared to the parent wild-type bacteria. In conclusion, our results suggest that P5 is important for bacterial OM stability, which ultimately affects the membrane proteome and NTHi pathogenesis.
Keywords: NTHI; P5; adherence; extracellular matrix; peptidoglycan; serum resistance; virulence.
Copyright © 2023 Su, Kadari, Straw, Janoušková, Jonsson, Thofte, Jalalvand, Matuschek, Sandblad, Végvári, Zubarev and Riesbeck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
LytM proteins play a crucial role in cell separation, outer membrane composition, and pathogenesis in nontypeable Haemophilus influenzae.mBio. 2015 Feb 24;6(2):e02575. doi: 10.1128/mBio.02575-14. mBio. 2015. PMID: 25714719 Free PMC article.
-
Defining the Binding Region in Factor H to Develop a Therapeutic Factor H-Fc Fusion Protein against Non-Typeable Haemophilus influenzae.Front Cell Infect Microbiol. 2016 Apr 13;6:40. doi: 10.3389/fcimb.2016.00040. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27148489 Free PMC article.
-
Nontypeable Haemophilus influenzae P5 Binds Human C4b-Binding Protein, Promoting Serum Resistance.J Immunol. 2021 Sep 15;207(6):1566-1577. doi: 10.4049/jimmunol.2100105. Epub 2021 Aug 25. J Immunol. 2021. PMID: 34433620 Free PMC article.
-
The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae.Front Immunol. 2018 Nov 5;9:2530. doi: 10.3389/fimmu.2018.02530. eCollection 2018. Front Immunol. 2018. PMID: 30455693 Free PMC article. Review.
-
The adhesins of non-typeable Haemophilus influenzae.Expert Rev Anti Infect Ther. 2018 Mar;16(3):187-196. doi: 10.1080/14787210.2018.1438263. Epub 2018 Feb 21. Expert Rev Anti Infect Ther. 2018. PMID: 29415569 Review.
Cited by
-
Lactoferrin impairs pathogen virulence through its proteolytic activity.Front Vet Sci. 2024 Aug 8;11:1428156. doi: 10.3389/fvets.2024.1428156. eCollection 2024. Front Vet Sci. 2024. PMID: 39176399 Free PMC article. Review.
-
Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions.Antibiotics (Basel). 2023 Dec 28;13(1):32. doi: 10.3390/antibiotics13010032. Antibiotics (Basel). 2023. PMID: 38247591 Free PMC article. Review.
References
-
- Atack J. M., Day C. J., Poole J., Brockman K. L., Bakaletz L. O., Barenkamp S. J., et al. . (2018). The HMW2 adhesin of non-typeable Haemophilus influenzae is a human-adapted lectin that mediates high-affinity binding to 2-6 linked n-acetylneuraminic acid glycans. Biochem. Biophys. Res. Commun. 503 (2), 1103–1107. doi: 10.1016/j.bbrc.2018.06.126 - DOI - PMC - PubMed
-
- Avadhanula V., Rodriguez C. A., Ulett G. C., Bakaletz L. O., Adderson E. E. (2006). Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74 (2), 830–838. doi: 10.1128/IAI.74.2.830-838.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

