Interrogation of an Enzyme Library Reveals the Catalytic Plasticity of Naturally Evolved [4+2] Cyclases
- PMID: 37305956
- PMCID: PMC10946715
- DOI: 10.1002/cbic.202300382
Interrogation of an Enzyme Library Reveals the Catalytic Plasticity of Naturally Evolved [4+2] Cyclases
Abstract
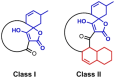
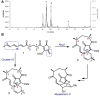
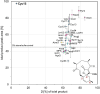
Stereoselective carbon-carbon bond forming reactions are quintessential transformations in organic synthesis. One example is the Diels-Alder reaction, a [4+2] cycloaddition between a conjugated diene and a dienophile to form cyclohexenes. The development of biocatalysts for this reaction is paramount for unlocking sustainable routes to a plethora of important molecules. To obtain a comprehensive understanding of naturally evolved [4+2] cyclases, and to identify hitherto uncharacterised biocatalysts for this reaction, we constructed a library comprising forty-five enzymes with reported or predicted [4+2] cycloaddition activity. Thirty-one library members were successfully produced in recombinant form. In vitro assays employing a synthetic substrate incorporating a diene and a dienophile revealed broad-ranging cycloaddition activity amongst these polypeptides. The hypothetical protein Cyc15 was found to catalyse an intramolecular cycloaddition to generate a novel spirotetronate. The crystal structure of this enzyme, along with docking studies, establishes the basis for stereoselectivity in Cyc15, as compared to other spirotetronate cyclases.
Keywords: biosynthetic gene cluster; cyclases; enzyme screening; intra-molecular Diels-Alder reaction; polyketides.
© 2023 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Oluwasanmi A., Hoskins C., Int. J. Pharm. 2021, 604, 120727; - PubMed
-
- Houk K. N., Liu F., Yang Z., Seeman J. I., Angew. Chem. Int. Ed. 2021, 60, 12660–12681; - PubMed
-
- Wang Y., Wu Q., Chen J., Liang F., Prog. Chem. 2022, 34, 474–486;
-
- Nicolaou K. C., Snyder S. A., Montagnon T., Vassilikogiannakis G., Angew. Chem. Int. Ed. 2002, 41, 1668–1698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

