P2Y12 inhibitor monotherapy versus dual antiplatelet therapy in patients with acute coronary syndromes undergoing coronary stenting: rationale and design of the NEOMINDSET Trial
- PMID: 37306039
- PMCID: PMC10333918
- DOI: 10.4244/EIJ-D-23-00125
P2Y12 inhibitor monotherapy versus dual antiplatelet therapy in patients with acute coronary syndromes undergoing coronary stenting: rationale and design of the NEOMINDSET Trial
Abstract
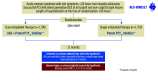
Dual antiplatelet therapy (DAPT) is currently the standard of care after percutaneous coronary intervention (PCI). Recent studies suggest that reducing DAPT to 1-3 months followed by an aspirin-free single antiplatelet therapy (SAPT) strategy with a potent P2Y12 inhibitor is safe and associated with less bleeding. However, to date, no randomised trial has tested the impact of initiating SAPT immediately after PCI, particularly in patients with acute coronary syndromes (ACS). NEOMINDSET is a multicentre, randomised, open-label trial with a blinded outcome assessment designed to compare SAPT versus DAPT in 3,400 ACS patients undergoing PCI with the latest-generation drug-eluting stents (DES). After successful PCI and up to 4 days following hospital admission, patients are randomised to receive SAPT with a potent P2Y12 inhibitor (ticagrelor or prasugrel) or DAPT (aspirin plus a potent P2Y12 inhibitor) for 12 months. Aspirin is discontinued immediately after randomisation in the SAPT group. The choice between ticagrelor and prasugrel is at the investigator's discretion. The primary hypothesis is that SAPT will be non-inferior to DAPT with respect to the composite endpoint of all-cause mortality, stroke, myocardial infarction or urgent target vessel revascularisation, but superior to DAPT on rates of bleeding defined by Bleeding Academic Research Consortium 2, 3 or 5 criteria. NEOMINDSET is the first study that is specifically designed to test SAPT versus DAPT immediately following PCI with DES in ACS patients. This trial will provide important insights on the efficacy and safety of withdrawing aspirin in the early phase of ACS. (ClinicalTrials.gov: NCT04360720).
Conflict of interest statement
R.H.M.Furtado reports personal fees from AstraZeneca, Apsen, Bayer, and Servier; and research grants from AstraZeneca, Novartis, Amgen, Brainfarma, the Brazilian Ministry of Health, and the University Health Network. J.C.Nicolau has received research grants from Amgen, AstraZeneca, Bayer, CSL Behring, Daiichi Sankyo, Dalcor, Esperion, Janssen, Novartis, Novo Nordisk, Sanofi, and Vifor; and is the recipient of ascholarship from the Brazilian National Council for Scientific and Technological Development (CNPq #303448/2021-0). R.D.Lopes reports research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, and Pfizer; consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, and Portola. M.Valgimigli reports personal fees from AstraZeneca; grants and personal fees from Terumo; personal fees from Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Bayer, CoreFLOW, Idorsia Pharmaceuticals Ltd, Universität Basel, Dept. Klinische Forschung, Vifor, Bristol-Myers Squibb, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, and PhaseBio, outside the submitted work.D.J. Angiolillo reports consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Ventura, outside the present work. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. P.W. Serruys declares consultancy for SMT, Philips, Novartis, Meril Life, and Xeltis. O.Berwanger reports consultancy fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis Pharma, and Pfizer. P.A.Lemos reports institutional research funding from, and/or being an unpaid advisory board member, and/or unpaid member of the steering/executive/data safety and monitoring group of trials, and/or unpaid interventional proctor for Abbott, Corindus, Scitech, Boston Scientific, and Flouit, but has not received personal payments from pharmaceutical companies or device manufacturers; being part of Argonauts, an innovation facilitator; and being partially supported by agrant from The National Council for Scientific and Technological Development (CNPq) - Brazil (grant number 306677/2019-9). The other authors have no conflicts of interest to declare.
Figures
References
-
- Bhatt DL, Lopes RD, Harrington RA. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA. 2022;327:662–75. - PubMed
-
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367. - PubMed
-
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–55. - PubMed
-
- Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han Yl, Pocock S, Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381:2032–42. - PubMed
-
- Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC MASTER DAPT Investigators. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med. 2021;385:1643–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

