Novel aldo-keto reductase 1C3 inhibitor affects androgen metabolism but not ovarian function in healthy women: a phase 1 study
- PMID: 37306288
- PMCID: PMC10376460
- DOI: 10.1093/ejendo/lvad063
Novel aldo-keto reductase 1C3 inhibitor affects androgen metabolism but not ovarian function in healthy women: a phase 1 study
Abstract
Objective: Aldo-keto reductase 1C3 (AKR1C3) has been postulated to be involved in androgen, progesterone, and estrogen metabolism. Aldo-keto reductase 1C3 inhibition has been proposed for treatment of endometriosis and polycystic ovary syndrome. Clinical biomarkers of target engagement, which can greatly facilitate drug development, have not yet been described for AKR1C3 inhibitors. Here, we analyzed pharmacodynamic data from a phase 1 study with a new selective AKR1C3 inhibitor, BAY1128688, to identify response biomarkers and assess effects on ovarian function.
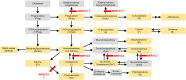
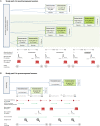
Design: In a multiple-ascending-dose placebo-controlled study, 33 postmenopausal women received BAY1128688 (3, 30, or 90 mg once daily or 60 mg twice daily) or placebo for 14 days. Eighteen premenopausal women received 60 mg BAY1128688 once or twice daily for 28 days.
Methods: We measured 17 serum steroids by liquid chromatography-tandem mass spectrometry, alongside analysis of pharmacokinetics, menstrual cyclicity, and safety parameters.
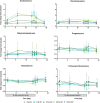
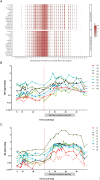
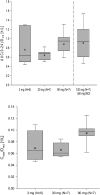
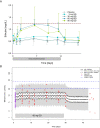
Results: In both study populations, we observed substantial, dose-dependent increases in circulating concentrations of the inactive androgen metabolite androsterone and minor increases in circulating etiocholanolone and dihydrotestosterone concentrations. In premenopausal women, androsterone concentrations increased 2.95-fold on average (95% confidence interval: 0.35-3.55) during once- or twice-daily treatment. Note, no concomitant changes in serum 17β-estradiol and progesterone were observed, and menstrual cyclicity and ovarian function were not altered by the treatment.
Conclusions: Serum androsterone was identified as a robust response biomarker for AKR1C3 inhibitor treatment in women. Aldo-keto reductase 1C3 inhibitor administration for 4 weeks did not affect ovarian function.ClinicalTrials.gov Identifier: NCT02434640; EudraCT Number: 2014-005298-36.
Keywords: androgens; androsterone; endometriosis; menstrual cycle; ovary; ovulation; polycystic ovary syndrome; randomized clinical trial; steroid metabolism.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Endocrinology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Desmond JC, Mountford JC, Drayson MT, et al. The aldo-keto reductase AKR1C3 is a novel suppressor of cell differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003;63(2):505–512. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

