Comparison of novel ventricular pacing strategies using an electro-mechanical simulation platform
- PMID: 37306315
- PMCID: PMC10259067
- DOI: 10.1093/europace/euad144
Comparison of novel ventricular pacing strategies using an electro-mechanical simulation platform
Abstract
Aims: Focus of pacemaker therapy is shifting from right ventricular (RV) apex pacing (RVAP) and biventricular pacing (BiVP) to conduction system pacing. Direct comparison between the different pacing modalities and their consequences to cardiac pump function is difficult, due to the practical implications and confounding variables. Computational modelling and simulation provide the opportunity to compare electrical, mechanical, and haemodynamic consequences in the same virtual heart.
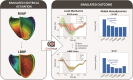
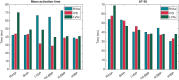
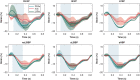
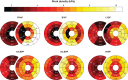
Methods and results: Using the same single cardiac geometry, electrical activation maps following the different pacing strategies were calculated using an Eikonal model on a three-dimensional geometry, which were then used as input for a lumped mechanical and haemodynamic model (CircAdapt). We then compared simulated strain, regional myocardial work, and haemodynamic function for each pacing strategy. Selective His-bundle pacing (HBP) best replicated physiological electrical activation and led to the most homogeneous mechanical behaviour. Selective left bundle branch (LBB) pacing led to good left ventricular (LV) function but significantly increased RV load. RV activation times were reduced in non-selective LBB pacing (nsLBBP), reducing RV load but increasing heterogeneity in LV contraction. LV septal pacing led to a slower LV and more heterogeneous LV activation than nsLBBP, while RV activation was similar. BiVP led to a synchronous LV-RV, but resulted in a heterogeneous contraction. RVAP led to the slowest and most heterogeneous contraction. Haemodynamic differences were small compared to differences in local wall behaviour.
Conclusion: Using a computational modelling framework, we investigated the mechanical and haemodynamic outcome of the prevailing pacing strategies in hearts with normal electrical and mechanical function. For this class of patients, nsLBBP was the best compromise between LV and RV function if HBP is not possible.
Keywords: Computational modelling; Conduction system pacing; Haemodynamics; Mechanics.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.L. has received research grants from Medtronic. F.W.P. has received research grants from Medtronic, Abbott, Microport CRM, and Biotronik. K.V. has received research grants from Medtronic, Abbott and has a consultancy agreement with Medtronic and Abbott. The remaining authors have nothing to disclose.
Figures




References
-
- Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His–Purkinje activation. Circulation 2000;101:869–77. - PubMed
-
- Ponnusamy SS, Arora V, Namboodiri N, Kumar V, Kapoor A, Vijayaraman P. Left bundle branch pacing: a comprehensive review. J Cardiovasc Electrophysiol 2020;31:2462–73. - PubMed
-
- Huang W, Su L, Wu S, Xu L, Xiao F, Zhou Xet al. . A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–.e3. - PubMed
-
- Prinzen FW, Oosterhout MPM, Vanagt WYR, Storm C, Reneman RS. Optimization of ventricular function by improving the activation sequence during ventricular pacing. Pacing Clin Electrophysiol 1998;21:2256–60. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

