Pharmacogenetic interactions of efavirenz or rifampin and isoniazid with levonorgestrel emergency contraception during treatment of HIV or tuberculosis
- PMID: 37306344
- PMCID: PMC10309098
- DOI: 10.1097/FPC.0000000000000501
Pharmacogenetic interactions of efavirenz or rifampin and isoniazid with levonorgestrel emergency contraception during treatment of HIV or tuberculosis
Abstract
Objective: In AIDS Clinical Trials Group study A5375, a pharmacokinetic trial of levonorgestrel emergency contraception, double-dose levonorgestrel (3 mg, versus standard dose 1.5 mg) offset the induction effects of efavirenz or rifampin on plasma levonorgestrel exposure over 8 h post-dose (AUC 0-8h ). We characterized the pharmacogenetics of these interactions.
Methods: Cisgender women receiving efavirenz- or dolutegravir-based HIV therapy, or on isoniazid-rifampin for tuberculosis, were followed after a single oral dose of levonorgestrel. Linear regression models, adjusted for BMI and age, characterized associations of CYP2B6 and NAT2 genotypes (which affect plasma efavirenz and isoniazid exposure, respectively) with levonorgestrel pharmacokinetic parameters.
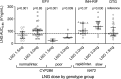
Results: Of 118 evaluable participants, 17 received efavirenz/levonorgestrel 1.5 mg, 35 efavirenz/levonorgestrel 3 mg, 34 isoniazid-rifampin/levonorgestrel 3 mg, and 32 (control group) dolutegravir/levonorgestrel 1.5 mg. There were 73 Black and 33 Asian participants. Regardless of genotype, women on efavirenz and isoniazid-rifampin had higher levonorgestrel clearance. In the efavirenz/levonorgestrel 3 mg group, CYP2B6 normal/intermediate metabolizers had levonorgestrel AUC 0-8h values similar to controls, while CYP2B6 poor metabolizers had AUC 0-8h values of 40% lower than controls. In the isoniazid-rifampin group, NAT2 rapid/intermediate acetylators had levonorgestrel AUC 0-8h values similar to controls, while NAT2 slow acetylators had AUC 0-8h values 36% higher than controls.
Conclusion: CYP2B6 poor metabolizer genotypes exacerbate the efavirenz-levonorgestrel interaction, likely by increased CYP3A induction with higher efavirenz exposure, making the interaction more difficult to overcome. NAT2 slow acetylator genotypes attenuate the rifampin-levonorgestrel interaction, likely by increased CYP3A inhibition with higher isoniazid exposure.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Scarsi: Organon, LLC. Research support paid to her institution. For the remaining authors, there are no conflicts of interest.
Figures

References
-
- World Health Organization. Global Tuberculosis Report 2022. 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa.... [Accessed 30 January 2023].
-
- World Health Organization. Tuberculosis in women. 2018. https://www.who.int/publications/m/item/tuberculosis-in-women. [Accessed 12 August 2022].
-
- Pillay T, Sturm AW, Khan M, Adhikari M, Moodley J, Connolly C, et al. . Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuberc Lung Dis 2004; 8:59–69. - PubMed
-
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. . U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103. - PubMed
-
- World Health Organization. Emergency contraception. 2021. https://www.who.int/news-room/fact-sheets/detail/emergency-contraception. [Accessed 5 April 2022].
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI068619/AI/NIAID NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UM1 AI069521/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- U01 AI068633/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 TR004406/TR/NCATS NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- R01 HD085887/HD/NICHD NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- U01 AI069399/AI/NIAID NIH HHS/United States
- R25 AI164610/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

