Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens
- PMID: 37306514
- PMCID: PMC10470525
- DOI: 10.1128/mbio.00360-23
Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens
Abstract
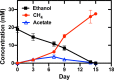
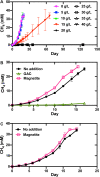
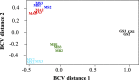
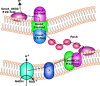
Methanothrix is widely distributed in natural and artificial anoxic environments and plays a major role in global methane emissions. It is one of only two genera that can form methane from acetate dismutation and through participation in direct interspecies electron transfer (DIET) with exoelectrogens. Although Methanothrix is a significant member of many methanogenic communities, little is known about its physiology. In this study, transcriptomics helped to identify potential routes of electron transfer during DIET between Geobacter metallireducens and Methanothrix thermoacetophila. Additions of magnetite to cultures significantly enhanced growth by acetoclastic methanogenesis and by DIET, while granular activated carbon (GAC) amendments impaired growth. Transcriptomics suggested that the OmaF-OmbF-OmcF porin complex and the octaheme outer membrane c-type cytochrome encoded by Gmet_0930, were important for electron transport across the outer membrane of G. metallireducens during DIET with Mx. thermoacetophila. Clear differences in the metabolism of Mx. thermoacetophila when grown via DIET or acetate dismutation were not apparent. However, genes coding for proteins involved in carbon fixation, the sheath fiber protein MspA, and a surface-associated quinoprotein, SqpA, were highly expressed in all conditions. Expression of gas vesicle genes was significantly lower in DIET- than acetate-grown cells, possibly to facilitate better contact between membrane-associated redox proteins during DIET. These studies reveal potential electron transfer mechanisms utilized by both Geobacter and Methanothrix during DIET and provide important insights into the physiology of Methanothrix in anoxic environments. IMPORTANCE Methanothrix is a significant methane producer in a variety of methanogenic environments including soils and sediments as well as anaerobic digesters. Its abundance in these anoxic environments has mostly been attributed to its high affinity for acetate and its ability to grow by acetoclastic methanogenesis. However, Methanothrix species can also generate methane by directly accepting electrons from exoelectrogenic bacteria through direct interspecies electron transfer (DIET). Methane production through DIET is likely to further increase their contribution to methane production in natural and artificial environments. Therefore, acquiring a better understanding of DIET with Methanothrix will help shed light on ways to (i) minimize microbial methane production in natural terrestrial environments and (ii) maximize biogas formation by anaerobic digesters treating waste.
Keywords: Geobacter; Methanothrix; acetate; archaea; cytochromes; direct interspecies electron transfer (DIET); granular activated carbon (GAC); magnetite; methane.
Figures


Similar articles
-
Metatranscriptomic Evidence for Direct Interspecies Electron Transfer between Geobacter and Methanothrix Species in Methanogenic Rice Paddy Soils.Appl Environ Microbiol. 2017 Apr 17;83(9):e00223-17. doi: 10.1128/AEM.00223-17. Print 2017 May 1. Appl Environ Microbiol. 2017. PMID: 28258137 Free PMC article.
-
Geobacter Strains Expressing Poorly Conductive Pili Reveal Constraints on Direct Interspecies Electron Transfer Mechanisms.mBio. 2018 Jul 10;9(4):e01273-18. doi: 10.1128/mBio.01273-18. mBio. 2018. PMID: 29991583 Free PMC article.
-
Mechanisms for Electron Uptake by Methanosarcina acetivorans during Direct Interspecies Electron Transfer.mBio. 2021 Oct 26;12(5):e0234421. doi: 10.1128/mBio.02344-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607451 Free PMC article.
-
Syntrophy Goes Electric: Direct Interspecies Electron Transfer.Annu Rev Microbiol. 2017 Sep 8;71:643-664. doi: 10.1146/annurev-micro-030117-020420. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697668 Review.
-
Putative Extracellular Electron Transfer in Methanogenic Archaea.Front Microbiol. 2021 Mar 22;12:611739. doi: 10.3389/fmicb.2021.611739. eCollection 2021. Front Microbiol. 2021. PMID: 33828536 Free PMC article. Review.
Cited by
-
Crucial roles of intracellular cyclic di-GMP in impacting the genes important for extracellular electron transfer by Geobacter metallireducens.Appl Environ Microbiol. 2025 Jul 23;91(7):e0072725. doi: 10.1128/aem.00727-25. Epub 2025 Jun 4. Appl Environ Microbiol. 2025. PMID: 40464557 Free PMC article.
-
Magnetite drives microbial community restructuring and stimulates aceticlastic methanogenesis of type II Methanosarcina in mangrove sediments.Microbiome. 2025 Jul 26;13(1):174. doi: 10.1186/s40168-025-02157-z. Microbiome. 2025. PMID: 40713905 Free PMC article.
-
Sheaths are diverse and abundant cell surface layers in archaea.ISME J. 2024 Jan 8;18(1):wrae225. doi: 10.1093/ismejo/wrae225. ISME J. 2024. PMID: 39499655 Free PMC article.
-
Anaerobic digestion of microalgae: microbial response and recovery after organic loading disturbances.mSystems. 2025 Mar 18;10(3):e0167424. doi: 10.1128/msystems.01674-24. Epub 2025 Feb 27. mSystems. 2025. PMID: 40013791 Free PMC article.
-
Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer.Int J Mol Sci. 2025 Apr 28;26(9):4195. doi: 10.3390/ijms26094195. Int J Mol Sci. 2025. PMID: 40362433 Free PMC article. Review.
References
-
- Holmes DE, Shrestha PM, Walker DJF, Dang Y, Nevin KP, Woodard TL, Lovley DR. 2017. Metatranscriptomic evidence for direct interspecies electron transfer between Geobacter and Methanothrix species in methanogenic rice paddy soils. Appl Environ Microbiol 83:e00223-17. doi:10.1128/AEM.00223-17 - DOI - PMC - PubMed
-
- Rotaru A-E, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler K, Wardman C, Nevin KP, Lovley DR. 2014. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7:408–415. doi:10.1039/C3EE42189A - DOI
-
- Angle JC, Morin TH, Solden LM, Narrowe AB, Smith GJ, Borton MA, Rey-Sanchez C, Daly RA, Mirfenderesgi G, Hoyt DW, Riley WJ, Miller CS, Bohrer G, Wrighton KC. 2017. Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat Commun 8:1567. doi:10.1038/s41467-017-01753-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

