Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus
- PMID: 37306558
- PMCID: PMC10259198
- DOI: 10.1002/14651858.CD013653.pub2
Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus
Abstract
Background: Hepatitis B virus (HBV)-human Immunodeficiency virus (HIV) co-infection promotes an aggressive disease course of HBV infection. In the only available non-Cochrane systematic review on antiviral therapy during pregnancy for prevention of mother-to-child transmission of HBV, none of the women studied had HBV-HIV co-infection but were either HBV- or HIV-seropositive. Treatment of HBV alone may develop HIV-strains that are resistant to non-nucleoside reverse transcriptase inhibitors. Accordingly, co-treatment of the HIV infection is recommended.
Objectives: To evaluate the benefits and harms of tenofovir-based antiviral combination regimens versus placebo, tenofovir alone, or non-tenofovir-based antiviral regimen either alone or in combination with HBV for the prevention of mother-to-child transmission of HBV in HIV-positive pregnant women co-infected with HBV.
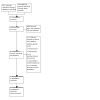
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials, MEDLINE Ovid, Embase Ovid, LILACS (Bireme), Science Citation Index Expanded (Web of Science), and Conference Proceedings Citation Index-Science (Web of Science) on 30 January 2023. We manually searched the reference lists of included trials, searched on-line trial registries, and contacted experts in the field and pharmaceutical companies for any further potential trials.
Selection criteria: We aimed to include randomised clinical trials comparing tenofovir-based antiviral combination regimens (anti-HIV regimen with lopinavir-ritonavir therapy, or any other antiviral therapy, and two drugs with activity against HBV, specifically, tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF), plus lamivudine or emtricitabine) with placebo alone, or tenofovir alone, or non-tenofovir-based antiviral regimen (zidovudine, lamivudine, telbivudine, emtricitabine, entecavir, lopinavir-ritonavir, or any other antiviral therapy) either alone or in combination with at least two other antivirals.
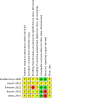
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Primary outcomes included all-cause infant mortality, proportion of infants with serious adverse events, proportion of infants with HBV mother-to-child transmission, all-cause maternal mortality, and proportion of mothers with serious adverse events. Secondary outcomes included proportion of infants with adverse events not considered serious, proportion of mothers with detectable HBV DNA (deoxyribonucleic acid) (before delivery), maternal hepatitis B e antigen (HBeAg) to HBe-antibody seroconversion (before delivery) and maternal adverse events not considered serious. We used RevMan Web to carry out analyses and presented results, where feasible, using a random-effects model and risk ratios (RR) with 95% confidence intervals (CIs). We performed sensitivity analysis. We assessed risk of bias using predefined domains, assessed the certainty of the evidence using GRADE, controlled risk of random errors with Trial Sequential Analysis, and presented outcome results in a summary of findings table.
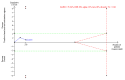
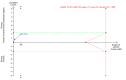
Main results: Five completed trials were included, of which four trials contributed data to one or more of the outcomes. They included a total of 533 participants randomised to tenofovir-based antiviral combination regimens (196 participants) versus control (337 participants). The control groups received non-tenofovir-based antiviral regimens either as zidovudine alone (three trials) or as a combination of zidovudine, lamivudine and lopinavir-ritonavir (five trials). None of the trials used placebo or tenofovir alone. All trials were at unclear risk of bias. Four trials used intention-to-treat analyses. In the remaining trial, two participants in the intervention group and two in the control group were lost to follow-up. However, the outcomes of these four participants were not described. Tenofovir-based antiviral combination regimen versus control We are very uncertain about the effect of a tenofovir-based antiviral combination regimen versus control on all-cause infant mortality (RR 2.24, 95% CI 0.72 to 6.96; participants = 132; trials = 1; very low-certainty evidence); proportion of infants with serious adverse events (RR 1.76, 95% CI 1.27 to 2.43; participants = 132; trials = 1; very low-certainty evidence), and proportion of mothers with serious adverse events (RR 0.90, 95% CI 0.62 to 1.32; participants = 262; trials = 2; very low-certainty evidence). No trial reported data on the proportion of infants with HBV mother-to-child transmission and all-cause maternal mortality. We are also very uncertain about the effect of tenofovir-based antiviral combination regimens versus control on the proportion of infants with adverse events not considered serious (RR 0.94, 95% CI 0.06 to 13.68; participants = 31; trials = 1; very low-certainty evidence), and proportion of mothers with detectable HBV DNA (before delivery) (RR 0.66, 95% CI 0.42 to 1.02; participants = 169; trials = 2; very low-certainty evidence). No trial reported data on maternal hepatitis B e antigen (HBeAg) to HBe-antibody seroconversion (before delivery) and maternal adverse events not considered serious. All trials received support from industry.
Authors' conclusions: We do not know what the effects of tenofovir-based antiviral combination regimens are on all-cause infant mortality, proportion of infants with serious adverse events and proportion of mothers with serious adverse events, proportion of infants with adverse events not considered serious, and proportion of mothers with detectable HBV DNA before delivery because the certainty of evidence was very low. Only one or two trials, with insufficient power, contributed data for analyses. We lack randomised clinical trials at low risk of systematic and random errors, and fully reporting all-cause infant mortality, serious adverse events and reporting on clinical and laboratory outcomes, such as infants with HBV mother-to-child transmission, all-cause maternal mortality, maternal hepatitis B e antigen (HBeAg) to HBe-antibody seroconversion before delivery and maternal adverse events not considered serious.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EOU: has no known conflicts of interest. AOU: has no known conflicts of interest. GUE: has no known conflicts of interest. UIN: has no known conflicts of interest. JII: has no known conflicts of interest. UAU: has no known conflicts of interest. HUO: has no known conflicts of interest.
Figures






Update of
References
References to studies included in this review
Bhattacharya 2020 {published data only}
-
- Bhattacharya D, Tierney C, Butler K, Moodley D, Govender V, Vhembo T, et al. Safety and HBV efficacy of antiretroviral therapy in women with HIV and HBV in the promoting maternal and infant survival everywhere study (PROMISE). Hepatology 2020;72(S1):494A-5A.
Fowler 2021 {published data only}
-
- Fowler MG, Pinilla M, Dadabhai S, Owor M, Stranix-Chibanda L, Flynn P, et al. Preterm birth, breastfeeding, antenatal ARV regimen and 24-month HIV-free survival. www.impaactnetwork.org/sites/default/files/inline-files/PROMISE_Fowler_C... (accessed 17 May 2022).
Kiweewa 2022 {published data only}
Kourtis 2018 {published data only}
-
- Kourtis AP, Wiener J, Wang L, Fan B, Shepherd JA, Chen L, et al. Tenofovir disoproxil fumarate use during pregnancy and infant bone health: the Tenofovir in Pregnancy Pilot Study. Pediatric Infectious Disease Journal 2018;37(11):e264-8. [Clinical Trial number: NCT01125696] [PMID: ] - PubMed
Wang 2016 {published data only}
-
- Wang L, Wiener J, Bulterys M, Wei X, Chen L, Liu W, et al. Hepatitis B virus (HBV) load response to 2 antiviral regimens, tenofovir/lamivudine and lamivudine, in HIV/ HBV-coinfected pregnant women in Guangxi, China: the Tenofovir in Pregnancy (TiP) Study. Journal of Infectious Diseases 2016;214(11):1695-9. [Clinical Trial number: NCT01125696] [PMID: ] - PMC - PubMed
References to studies excluded from this review
Baciu 2021 {published data only}
-
- Baciu L, Domuncu D, Domuncu T, Ciobanu AM, Voiosu T, Peltecu G, et al. Tenofovir disoproxil fumarate for preventing mother-to-child transmission of hepatitis B: a literature review. Clinical and Experimental Obstetetrics & Gynecology 2021;48(1):9-18.
Bhattacharya 2021 {published data only}
Bierhoff 2020 {published data only}
-
- Bierhoff M, Rijken MJ, Yotyingaphiram W, Pimanpanarak M, Van Vugt M, Angkurawaranon C, et al. Tenofovir for prevention of mother to child transmission of hepatitis B in migrant women in a resource-limited setting on the Thailand-Myanmar border: a commentary on challenges of implementation. International Journal for Equity In Health 2020;19(1):156. [PMID: ] - PMC - PubMed
Bukkems 2021 {published data only}
-
- Bukkems VE, Smolders EJ, Jourdain G, Burger DM, Colbers AP, Cressey TR. Effect of pregnancy and concomitant antiretrovirals on the pharmacokinetics of tenofovir in women with HIV receiving tenofovir disoproxil fumarate-based antiretroviral therapy versus women with HBV receiving tenofovir disoproxil fumarate monotherapy. Journal of Clinical Pharmacology 2021;61(3):388-93. [PMID: ] - PMC - PubMed
Hompe 2019 {published data only}
Jourdain 2016 {published data only}
-
- Jourdain G, Ngo-Giang-Huong N, Cressey TR, Hua L, Harrison L, Tierney C, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infectious Diseases 2016;16:393. [PMID: ] - PMC - PubMed
Jourdain 2018 {published data only}
Lin 2018 {published data only}
Mave 2014 {published data only}
Pan 2016 {published data only}
-
- Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. New England Journal of Medicine 2016;374(24):2324-34. [PMID: ] - PubMed
Sarkar 2018 {published data only}
-
- Sarkar J, Saha D, Bandyopadhyay B, Saha B, Chakravarty R, Guha SK. Lamivudine plus tenofovir versus lamivudine plus adefovir for the treatment of hepatitis B virus in HIV-coinfected patients, starting antiretroviral therapy. Indian Journal of Medical Microbiology 2018;36(2):217-23. [PMID: ] - PubMed
Additional references
AASLD 2018
-
- American Association for the Study of Liver Diseases (AASLD) guidelines of 2018. Practice Guidelines. www.aasld.org/practice-guidelines (accessed 22 December 2022).
Ahn 2010
Andreotti 2014
Atkins 2004
Ba 2019
Baheti 2011
Benhammou 2018
-
- Benhammou V, Tubiana R, Matheron S, Sellier P, Mandelbrot L, Chenadec JL, et al. HBV or HCV coinfection in HIV-1-infected pregnant women in France: prevalence and pregnancy outcomes. Journal of Acquired Immune Deficiency Syndromes 2018;77(5):439-50. [PMID: ] - PubMed
Benhamou 1999
-
- Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999;30(5):1302-6. [PMID: ] - PubMed
Boettiger 2016
Bosh 2018
Brown 2016
-
- Brown RS Jr, McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology 2016;63(1):319-33. [PMID: ] - PubMed
Butcher 2022
-
- Butcher NJ, Monsour A, Mew EJ, Chan AW, Moher D, Mayo-Wilson E, et al. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022;328(22):2252-64. - PubMed
Calcagno 2016
-
- Calcagno A, Cusato J, Marinaro L, Trentini L, Alcantarini C, Mussa M, et al. Clinical pharmacology of tenofovir clearance: a pharmacokinetic/pharmacogenetic study on plasma and urine. Pharmacogenomics Journal 2016;16(6):514-8. [PMID: ] - PubMed
Castellini 2018
De Clercq 2018
-
- De Clercq E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochemical Pharmacology 2018;153:2-11. [PMID: ] - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DeJesus 2018
-
- DeJesus E, Haas B, Segal-Maurer S, Ramgopal MN, Mills A, Margot N, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Research and Human Retroviruses 2018;34(4):337-42. [PMID: ] - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177–88. - PubMed
Djaogol 2019
Dore 2004
-
- Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. Journal of Infectious Diseases 2004;189(7):1185-92. [PMID: ] - PubMed
Eke 2017
Eke 2020
Eleje 2014
-
- Eleje G, Ele P, Okocha E, Iloduba U. Epidemiology and clinical parameters of adult human immunodeficiency virus/acquired immunodeficiency syndrome at the initiation of antiretroviral therapy in South Eastern Nigeria. Annals of Medical and Health Sciences Research 2014;4(2):217-21. [PMID: ] - PMC - PubMed
Eleje 2018
-
- Eleje GU, Edokwe ES, Ikechebelu JI, Onubogu CU, Ugochukwu EF, Okam PC, et al. Mother-to-child transmission of human immunodeficiency virus (HIV) among HIV-infected pregnant women on highly active anti-retroviral therapy with premature rupture of membranes at term. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(2):184-90. [PMID: ] - PubMed
Eleje 2021
Eleje 2022
-
- Eleje GU, Onubogu CU, Fiebai PO, Mbachu II, Akaba GO, Loto OM, et al. Mother-to-child transmission of human immunodeficiency virus, hepatitis B virus and hepatitis C virus among pregnant women with single, dual or triplex infections of human immunodeficiency virus, hepatitis B virus and hepatitis C virus in Nigeria: a systematic review and meta-analysis. SAGE Open Medicine 2022;10:20503121221095411. [PMID: PMID: 35509955] - PMC - PubMed
Floridia 2017
-
- Floridia M, Masuelli G, Tamburrini E, Spinillo A, Simonazzi G, Guaraldi G, et al. HBV coinfection is associated with reduced CD4 response to antiretroviral treatment in pregnancy. HIV Clinical Trials 2017;18(2):54-9. [PMID: ] - PubMed
Fowler 2016
Frempong 2019
Gallant 2016
-
- Gallant J, Brunetta J, Crofoot G, Benson P, Mills A, Brinson C, et al. Brief report: efficacy and safety of switching to a single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide in HIV-1/hepatitis B coinfected adults. Journal of Acquired Immune Deficiency Syndromes 2016;73(3):294-8. [PMID: ] - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed 22 December 2022). Available at gradepro.org.
Hagins 2018
-
- Hagins D, Orkin C, Daar ES, Mills A, Brinson C, DeJesus E, et al. Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Medicine 2018;19(10):724-33. [PMID: ] - PMC - PubMed
Hall 2011
-
- Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. American Journal of Kidney Diseases 2011;57(5):773-80. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Higgins 2019b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoff 2001
-
- Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of anti-human immunodeficiency virus regimens. Clinical Infectious Diseases 2001;32(6):963-9. [PMID: ] - PubMed
Hoffmann 2007
-
- Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infectious Diseases 2007;7(6):402-9. [PMID: ] - PubMed
Huhn 2017
-
- Huhn GD, Tebas P, Gallant J, Wilkin T, Cheng A, Yan M, et al. A randomized, open-label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment-experienced HIV-1-infected adults. Journal of Acquired Immune Deficiency Syndromes 2017;74(2):193-200. [PMID: ] - PMC - PubMed
ICH‐GCP 2016
-
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 22 December 2022).
Imberger 2016
Jakobsen 2014
Jibril 2013
Joe‐Ikechebelu 2019
Johnson 1999
-
- Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clinical Pharmacokinetics 1999;36(1):41-66. [PMID: ] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. - PubMed
Kourtis 2012
Li 2020
Lundh 2017
Luo 2018
Mave 2014
Mbougua 2010
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609–13. - PubMed
Molina 2018
-
- Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48-week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018;5(7):e357-65. [PMID: ] - PubMed
Moss 2014
Mpody 2019
Mu 2020
Naing 2018
Ogbuagu 2021
-
- Ogbuagu CN, Ogbuagu EN, Okoh EE, Olli UT, Okereke UC, Emelumadu OF, et al. Outcome of fixed dose combination of tenofovir, lamivudine and dolutegravir in rural HIV care facility in Nigeria. International Journal of Health Sciences and Research 2021;11(12):1-8.
Omotowo 2018
-
- Omotowo IB, Meka IA, Ijoma UN, Okoli VE, Obienu O, Nwagha T, et al. Uptake of hepatitis B vaccination and its determinants among health care workers in a tertiary health facility in Enugu, South-East, Nigeria. BMC Infectious Diseases 2018;18(1):288. [DOI: 10.1186/s12879-018-3191-9] [PMID: ] - DOI - PMC - PubMed
Ouyang 2009
Page 2019
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Podany 2018
Price 2013
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020.
Rough 2018
Saag 2006
-
- Saag MS. Emtricitabine, a new antiretroviral agent with activity against HIV and hepatitis B virus. Clinical Infectious Diseases 2006;42(1):126-31. [PMID: ] - PubMed
Sangaré 2009
-
- Sangaré L, Sombié R, Combasséré AW, Kouanda A, Kania D, Zerbo O, et al. Antenatal transmission of hepatitis B virus in an area of HIV moderate prevalence, Burkina Faso. Bulletin de la Societe de Pathologie Exotique 2009;102(4):226-9. - PubMed
Sarmati 2019
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. - PubMed
Savović 2018
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408–12. - PubMed
Seley‐Radtke 2018
Siegfried 2011
Siemieniuk 2017
Sterne 2011
-
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Stewart 2011
Storebø 2018
-
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] - DOI - PMC - PubMed
Sun 2014
Tavakolpour 2018
-
- Tavakolpour S, Darvishi M, Mirsafaei HS, Ghasemiadl M. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B infection during pregnancy: a systematic review. Infectious Diseases 2018;50(2):95-106. [PMID: ] - PubMed
Tengan 2017
Terrault 2016
Terrault 2018
Thorlund 2017
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more (accessed 22 December 2022).
Tourret 2013
TSA 2017 [Computer program]
-
- TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017. ctu.dk/tsa/downloads/.
Uglietti 2012
-
- Uglietti A, Zanaboni D, Gnarini M, Maserati R. Emtricitabine/tenofovir in the treatment of HIV infection: current PK/PD evaluation. Expert Opinion on Drug Metabolism & Toxicology 2012;8(10):1305-14. [PMID: ] - PubMed
Uyoh 2018
-
- Uyoh IS, Eleje GU, Onyegbule OA, Oguejiofor CB, Umeobika JC, Okaforcha EI. Correlates and prevalence of human immunodeficiency virus and hepatitis B virus co-infection in pregnancy. International Journal of Advances in Advances in Medical Sciences 2018;3(7):1-12.
Wang 2019
-
- Wang C, Yu S, Zhang Y, Zhang M, Lv L, Huang C, et al. Viral quasi species of hepatitis B virus in patients with YMDD mutation and lamivudine resistance may not predict the efficacy of lamivudine/adefovir rescue therapy. Experimental and Therapeutic Medicine 2019;17(4):2473-84. [PMID: ] - PMC - PubMed
Weitzel 2020
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64–75. - PubMed
Wetterslev 2009
Wetterslev 2017
Wood 2008
Xu 2013
Yi 2016
-
- Yi P, Chen R, Huang Y, Zhou RR, Fan XG. Management of mother-to-child transmission of hepatitis B virus: propositions and challenges. Journal of Clinical Virology 2016;77:32-9. [PMID: ] - PubMed
References to other published versions of this review
Ugwu 2020
-
- Ugwu EO, Eleje GU, Ugwu AO, Nwagha UI, Ikechebelu JI, Umeh UA, et al. Antivirals for prevention of hepatitis B virus mother‐to‐child transmission in human immunodeficiency virus positive pregnant women co‐infected with hepatitis B virus. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013653. [DOI: 10.1002/14651858.CD013653] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

