Four-Electron Reduction of O2 Using Distibines in the Presence of ortho-Quinones
- PMID: 37306561
- PMCID: PMC10863049
- DOI: 10.1021/jacs.3c02223
Four-Electron Reduction of O2 Using Distibines in the Presence of ortho-Quinones
Abstract
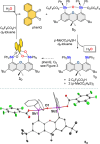
This study, which aims to identify atypical platforms for the reduction of dioxygen, describes the reaction of O2 with two distibines, namely, 4,5-bis(diphenylstibino)-2,7-di-tert-butyl-9,9-dimethylxanthene and 4,5-bis(diphenylstibino)-2,7-di-tert-butyl-9,9-dimethyldihydroacridine, in the presence of an ortho-quinone such as phenanthraquinone. The reaction proceeds by oxidation of the two antimony atoms to the + V state in concert with reductive cleavage of the O2 molecule. As confirmed by 18O labeling experiments, the two resulting oxo units combine with the ortho-quinone to form an α,α,β,β-tetraolate ligand that bridges the two antimony(V) centers. This process, which has been studied both experimentally and computationally, involves the formation of asymmetric, mixed-valent derivatives featuring a stibine as well as a catecholatostiborane formed by oxidative addition of the quinone to only one of the antimony centers. Under aerobic conditions, the catecholatostiborane moiety reacts with O2 to form a semiquinone/peroxoantimony intermediate, as supported by NMR spectroscopy in the case of the dimethyldihydroacridine derivative. These intermediates swiftly evolve into the symmetrical bis(antimony(V)) α,α,β,β-tetraolate complexes via low barrier processes. Finally, the controlled protonolysis and reduction of the bis(antimony(V)) α,α,β,β-tetraolate complex based on the 9,9-dimethylxanthene platform have been investigated and shown to regenerate the starting distibine and the ortho-quinone. More importantly, these last reactions also produce two equivalents of water as the product of O2 reduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Sengupta K.; Chatterjee S.; Dutta K.. Oxygen Reduction Reaction; Elsevier, 2022.
- Luckner M.Oxidoreductases and Oxygenases. Secondary Metabolism in Microorganisms, Plants and Animals; Springer Berlin Heidelberg: Berlin, Heidelberg, 1984; pp 88–103.
- Frey P. A.; Hegeman A. D.; Frey P. A.; Hegeman A. D.. Oxidases and Oxygenases. Enzymatic Reaction Mechanisms; Oxford University Press, 2007; p 0.
- Babcock G. T.; Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature 1992, 356, 301–309. 10.1038/356301a0. - DOI - PubMed
- Denisov I. G.; Makris T. M.; Sligar S. G.; Schlichting I. Structure and Chemistry of Cytochrome P450. Chem. Rev. 2005, 105, 2253–2278. 10.1021/cr0307143. - DOI - PubMed
- Kovaleva E. G.; Lipscomb J. D. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat. Chem. Biol. 2008, 4, 186–193. 10.1038/nchembio.71. - DOI - PMC - PubMed
- Solomon E. I.; Heppner D. E.; Johnston E. M.; Ginsbach J. W.; Cirera J.; Qayyum M.; Kieber-Emmons M. T.; Kjaergaard C. H.; Hadt R. G.; Tian L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. 10.1021/cr400327t. - DOI - PMC - PubMed
- Poulos T. L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. 10.1021/cr400415k. - DOI - PMC - PubMed
- Yoshikawa S.; Shimada A. Reaction Mechanism of Cytochrome c Oxidase. Chem. Rev. 2015, 115, 1936–1989. 10.1021/cr500266a. - DOI - PubMed
- Wikström M.; Krab K.; Sharma V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018, 118, 2469–2490. 10.1021/acs.chemrev.7b00664. - DOI - PMC - PubMed
-
- Solomon E. I.; Sundaram U. M.; Machonkin T. E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. 10.1021/cr950046o. - DOI - PubMed
- Costas M.; Mehn M. P.; Jensen M. P.; Que L. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004, 35, 939–986. 10.1002/chin.200421291. - DOI - PubMed
- Kryatov S. V.; Rybak-Akimova E. V.; Schindler S. Kinetics and Mechanisms of Formation and Reactivity of Non-heme Iron Oxygen Intermediates. Chem. Rev. 2005, 105, 2175–2226. 10.1021/cr030709z. - DOI - PubMed
- Huang X.; Groves J. T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. 10.1021/acs.chemrev.7b00373. - DOI - PMC - PubMed
- Fiedler A. T.; Fischer A. A. Oxygen activation by mononuclear Mn, Co, and Ni centers in biology and synthetic complexes. JBIC, J. Biol. Inorg. Chem. 2017, 22, 407–424. 10.1007/s00775-016-1402-7. - DOI - PubMed
-
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994, 269, 22459–22462. 10.1016/s0021-9258(17)31664-2. - DOI - PubMed
- Palfey B. A.; McDonald C. A. Control of catalysis in flavin-dependent monooxygenases. Arch. Biochem. Biophys. 2010, 493, 26–36. 10.1016/j.abb.2009.11.028. - DOI - PubMed
- Romero E.; Gómez Castellanos J. R.; Gadda G.; Fraaije M. W.; Mattevi A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem. Rev. 2018, 118, 1742–1769. 10.1021/acs.chemrev.7b00650. - DOI - PubMed
-
- Stahl S. S. Palladium-Catalyzed Oxidation of Organic Chemicals with O2. Science 2005, 309, 1824–1826. 10.1126/science.1114666. - DOI - PubMed
- Boisvert L.; Goldberg K. I. Reactions of Late Transition Metal Complexes with Molecular Oxygen. Acc. Chem. Res. 2012, 45, 899–910. 10.1021/ar2003072. - DOI - PubMed
- McCann S. D.; Stahl S. S. Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res. 2015, 48, 1756–1766. 10.1021/acs.accounts.5b00060. - DOI - PubMed
- Neu H. M.; Baglia R. A.; Goldberg D. P. A Balancing Act: Stability versus Reactivity of Mn(0) Complexes. Acc. Chem. Res. 2015, 48, 2754–2764. 10.1021/acs.accounts.5b00273. - DOI - PMC - PubMed
- Pegis M. L.; Wise C. F.; Martin D. J.; Mayer J. M. Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. 10.1021/acs.chemrev.7b00542. - DOI - PubMed
-
- Wood T. K.; Piers W. E.; Keay B. A.; Parvez M. 9-Boraanthracene Derivatives Stabilized by N-Heterocyclic Carbenes. Angew. Chem., Int. Ed. 2009, 48, 4009–4012. 10.1002/anie.200901217. - DOI - PubMed
- Porcel S.; Bouhadir G.; Saffon N.; Maron L.; Bourissou D. Reaction of Singlet Dioxygen with Phosphine-Borane Derivatives: From Transient Phosphine Peroxides to Crystalline Peroxoboronates. Angew. Chem., Int. Ed. 2010, 49, 6186–6189. 10.1002/anie.201000520. - DOI - PubMed
- Henthorn J. T.; Agapie T. Dioxygen Reactivity with a Ferrocene–Lewis Acid Pairing: Reduction to a Boron Peroxide in the Presence of Tris(pentafluorophenyl)borane. Angew. Chem., Int. Ed. 2014, 53, 12893–12896. 10.1002/anie.201408462. - DOI - PubMed
- Henthorn J. T.; Lin S.; Agapie T. Combination of Redox-Active Ligand and Lewis Acid for Dioxygen Reduction with π-Bound Molybdenum–Quinonoid Complexes. J. Am. Chem. Soc. 2015, 137, 1458–1464. 10.1021/ja5100405. - DOI - PubMed
- Wang T.; Kehr G.; Liu L.; Grimme S.; Daniliuc C. G.; Erker G. Selective Oxidation of an Active Intramolecular Amine/Borane Frustrated Lewis Pair with Dioxygen. J. Am. Chem. Soc. 2016, 138, 4302–4305. 10.1021/jacs.6b00325. - DOI - PubMed
- Tsurumaki E.; Sung J.; Kim D.; Osuka A. Stable Boron Peroxides with a Subporphyrinato Ligand. Angew. Chem., Int. Ed. 2016, 55, 2596–2599. 10.1002/anie.201511590. - DOI - PubMed
- Kong L.; Lu W.; Li Y.; Ganguly R.; Kinjo R. Azaborabutadienes: Synthesis by Metal-Free Carboboration of Nitriles and Utility as Building Blocks for B,N-Heterocycles. Angew. Chem., Int. Ed. 2016, 55, 14718–14722. 10.1002/anie.201608994. - DOI - PubMed
- Taylor J. W.; McSkimming A.; Guzman C. F.; Harman W. H. N-Heterocyclic Carbene-Stabilized Boranthrene as a Metal-Free Platform for the Activation of Small Molecules. J. Am. Chem. Soc. 2017, 139, 11032–11035. 10.1021/jacs.7b06772. - DOI - PubMed
- Tao X.; Daniliuc C. G.; Janka O.; Pöttgen R.; Knitsch R.; Hansen M. R.; Eckert H.; Lübbesmeyer M.; Studer A.; Kehr G.; Erker G. Reduction of Dioxygen by Radical/B(p-C6F4X)3 Pairs to Give Isolable Bis(borane)superoxide Compounds. Angew. Chem., Int. Ed. 2017, 56, 16641–16644. 10.1002/anie.201709309. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

