A Cysteine Residue of Human Cytomegalovirus vMIA Protein Plays a Crucial Role in Viperin Trafficking to Control Viral Infectivity
- PMID: 37306568
- PMCID: PMC10308886
- DOI: 10.1128/jvi.01874-22
A Cysteine Residue of Human Cytomegalovirus vMIA Protein Plays a Crucial Role in Viperin Trafficking to Control Viral Infectivity
Abstract
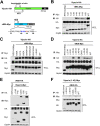
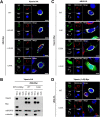
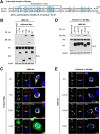
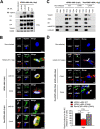
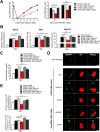
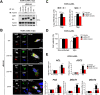
Viperin is a multifunctional interferon-inducible protein that is directly induced in cells by human cytomegalovirus (HCMV) infection. The viral mitochondrion-localized inhibitor of apoptosis (vMIA) interacts with viperin at the early stages of infection and translocates it from the endoplasmic reticulum to the mitochondria, where viperin modulates the cellular metabolism to increase viral infectivity. Viperin finally relocalizes to the viral assembly compartment (AC) at late stages of infection. Despite the importance of vMIA interactions with viperin during viral infection, their interacting residues are unknown. In the present study, we showed that cysteine residue 44 (Cys44) of vMIA and the N-terminal domain (amino acids [aa] 1 to 42) of viperin are necessary for their interaction and for the mitochondrial localization of viperin. In addition, the N-terminal domain of mouse viperin, which is structurally similar to that of human viperin, interacted with vMIA. This indicates that the structure, rather than the sequence composition, of the N-terminal domain of viperin, is required for the interaction with vMIA. Recombinant HCMV, in which Cys44 of vMIA was replaced by an alanine residue, failed to translocate viperin to the mitochondria at the early stages of infection and inefficiently relocalized it to the AC at late stages of infection, resulting in the impairment of viperin-mediated lipid synthesis and a reduction in viral replication. These data indicate that Cys44 of vMIA is therefore essential for the intracellular trafficking and function of viperin to increase viral replication. Our findings also suggest that the interacting residues of these two proteins are potential therapeutic targets for HCMV-associated diseases. IMPORTANCE Viperin traffics to the endoplasmic reticulum (ER), mitochondria, and viral assembly compartment (AC) during human cytomegalovirus (HCMV) infection. Viperin has antiviral activity at the ER and regulates cellular metabolism at the mitochondria. Here, we show that Cys44 of HCMV vMIA protein and the N-terminal domain (aa 1 to 42) of viperin are necessary for their interaction. Cys44 of vMIA also has a critical role for viperin trafficking from the ER to the AC via the mitochondria during viral infection. Recombinant HCMV expressing a mutant vMIA Cys44 has impaired lipid synthesis and viral infectivity, which are attributed to mislocalization of viperin. Cys44 of vMIA is essential for the trafficking and function of viperin and may be a therapeutic target for HCMV-associated diseases.
Keywords: HCMV; interacting residues; interferon-inducible protein; intracellular trafficking; vMIA; viperin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity.Science. 2011 May 27;332(6033):1093-7. doi: 10.1126/science.1202007. Epub 2011 Apr 28. Science. 2011. PMID: 21527675
-
The human cytomegalovirus protein UL37 exon 1 associates with internal lipid rafts.J Virol. 2011 Mar;85(5):2100-11. doi: 10.1128/JVI.01830-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177823 Free PMC article.
-
Human cytomegalovirus inhibits apoptosis by proteasome-mediated degradation of Bax at endoplasmic reticulum-mitochondrion contacts.J Virol. 2013 May;87(10):5657-68. doi: 10.1128/JVI.00145-13. Epub 2013 Mar 13. J Virol. 2013. PMID: 23487455 Free PMC article.
-
Superresolution imaging of viral protein trafficking.Med Microbiol Immunol. 2015 Jun;204(3):449-60. doi: 10.1007/s00430-015-0395-0. Epub 2015 Feb 28. Med Microbiol Immunol. 2015. PMID: 25724304 Free PMC article. Review.
-
vMIA, a viral inhibitor of apoptosis targeting mitochondria.Biochimie. 2002 Feb-Mar;84(2-3):177-85. doi: 10.1016/s0300-9084(02)01367-6. Biochimie. 2002. PMID: 12022948 Review.
Cited by
-
Crucial Roles of RSAD2/viperin in Immunomodulation, Mitochondrial Metabolism and Autoimmune Diseases.Inflammation. 2025 Apr;48(2):520-540. doi: 10.1007/s10753-024-02076-5. Epub 2024 Jun 23. Inflammation. 2025. PMID: 38909344 Review.
-
Viperin: A Multifunctional Protein in Antiviral Immunity and Disease Pathogenesis.Pathogens. 2025 May 21;14(5):510. doi: 10.3390/pathogens14050510. Pathogens. 2025. PMID: 40430829 Free PMC article. Review.
-
The battle between host antiviral innate immunity and immune evasion by cytomegalovirus.Cell Mol Life Sci. 2024 Aug 9;81(1):341. doi: 10.1007/s00018-024-05369-y. Cell Mol Life Sci. 2024. PMID: 39120730 Free PMC article. Review.
-
Cytomegalovirus Biology Viewed Through a Cell Death Suppression Lens.Viruses. 2024 Nov 23;16(12):1820. doi: 10.3390/v16121820. Viruses. 2024. PMID: 39772130 Free PMC article. Review.
References
-
- Meakins JL. 1983. Clinical approach to infection in the compromised host. Ann Surg 197:491–491.
-
- Warnatsch A. 2013. Impact of proteasomal immune adaptation on the early immune response to viral infection. PhD thesis. Kings College London, London, UK.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

