Genetic Analysis Reveals a Requirement for the Hybrid Sensor Kinase RscS in para-Aminobenzoic Acid/Calcium-Induced Biofilm Formation by Vibrio fischeri
- PMID: 37306594
- PMCID: PMC10367588
- DOI: 10.1128/jb.00075-23
Genetic Analysis Reveals a Requirement for the Hybrid Sensor Kinase RscS in para-Aminobenzoic Acid/Calcium-Induced Biofilm Formation by Vibrio fischeri
Abstract
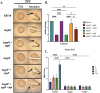
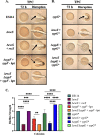
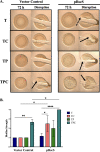
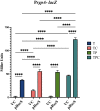
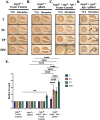
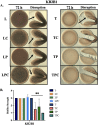
The marine bacterium Vibrio fischeri initiates symbiotic colonization of its squid host, Euprymna scolopes, by forming and dispersing from a biofilm dependent on the
Keywords: RscS; Vibrio fischeri; biofilms; calcium signaling; pABA; sensor kinase; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
A single point mutation is sufficient to drive syp-dependent biofilm formation and promote colonization by Vibrio fischeri.J Bacteriol. 2025 Aug 21;207(8):e0013125. doi: 10.1128/jb.00131-25. Epub 2025 Jul 14. J Bacteriol. 2025. PMID: 40657914 Free PMC article.
-
Vibrio fischeri: a model for host-associated biofilm formation.J Bacteriol. 2024 Feb 22;206(2):e0037023. doi: 10.1128/jb.00370-23. Epub 2024 Jan 25. J Bacteriol. 2024. PMID: 38270381 Free PMC article. Review.
-
Para-Aminobenzoic Acid, Calcium, and c-di-GMP Induce Formation of Cohesive, Syp-Polysaccharide-Dependent Biofilms in Vibrio fischeri.mBio. 2021 Oct 26;12(5):e0203421. doi: 10.1128/mBio.02034-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607467 Free PMC article.
-
Discovery of Calcium as a Biofilm-Promoting Signal for Vibrio fischeri Reveals New Phenotypes and Underlying Regulatory Complexity.J Bacteriol. 2018 Jul 10;200(15):e00016-18. doi: 10.1128/JB.00016-18. Print 2018 Aug 1. J Bacteriol. 2018. PMID: 29463601 Free PMC article.
-
An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri.Mol Microbiol. 2009 Nov;74(4):782-9. doi: 10.1111/j.1365-2958.2009.06899.x. Epub 2009 Oct 8. Mol Microbiol. 2009. PMID: 19818022 Free PMC article. Review.
Cited by
-
LitR and its quorum-sensing regulators modulate biofilm formation by Vibrio fischeri.J Bacteriol. 2025 Feb 20;207(2):e0047624. doi: 10.1128/jb.00476-24. Epub 2025 Jan 29. J Bacteriol. 2025. PMID: 39878466 Free PMC article.
-
A single point mutation is sufficient to drive syp-dependent biofilm formation and promote colonization by Vibrio fischeri.J Bacteriol. 2025 Aug 21;207(8):e0013125. doi: 10.1128/jb.00131-25. Epub 2025 Jul 14. J Bacteriol. 2025. PMID: 40657914 Free PMC article.
-
Vibrio fischeri: a model for host-associated biofilm formation.J Bacteriol. 2024 Feb 22;206(2):e0037023. doi: 10.1128/jb.00370-23. Epub 2024 Jan 25. J Bacteriol. 2024. PMID: 38270381 Free PMC article. Review.
-
The biofilm community resurfaces: new findings and post-pandemic progress.J Bacteriol. 2023 Oct 26;205(10):e0016623. doi: 10.1128/jb.00166-23. Epub 2023 Sep 27. J Bacteriol. 2023. PMID: 37756166 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

