Add-on Rehmannia-6-Based Chinese Medicine in Type 2 Diabetes and CKD: A Multicenter Randomized Controlled Trial
- PMID: 37307005
- PMCID: PMC10564374
- DOI: 10.2215/CJN.0000000000000199
Add-on Rehmannia-6-Based Chinese Medicine in Type 2 Diabetes and CKD: A Multicenter Randomized Controlled Trial
Abstract
Background: Diabetes is the leading cause of CKD and kidney failure. We assessed the real-world effectiveness of Rehmannia-6-based Chinese medicine treatment, the most used Chinese medicine formulation, on the change in eGFR and albuminuria in patients with diabetes and CKD with severely increased albuminuria.
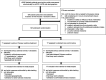
Methods: In this randomized, assessor-blind, standard care-controlled, parallel, multicenter trial, 148 adult patients from outpatient clinics with type 2 diabetes, an eGFR of 30-90 ml/min per 1.73 m 2 , and a urine albumin-to-creatinine ratio (UACR) of 300-5000 mg/g were randomized 1:1 to a 48-week add-on protocolized Chinese medicine treatment program (using Rehmannia-6-based formulations in the granule form taken orally) or standard care alone. Primary outcomes were the slope of change in eGFR and UACR between baseline and end point (48 weeks after randomization) in the intention-to-treat population. Secondary outcomes included safety and the change in biochemistry, biomarkers, and concomitant drug use.
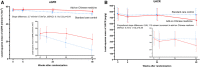
Results: The mean age, eGFR, and UACR were 65 years, 56.7 ml/min per 1.73 m 2 , and 753 mg/g, respectively. Ninety-five percent ( n =141) of end point primary outcome measures were retrievable. For eGFR, the estimated slope of change was -2.0 (95% confidence interval [CI], -0.1 to -3.9) and -4.7 (95% CI, -2.9 to -6.5) ml/min per 1.73 m 2 in participants treated with add-on Chinese medicine or standard care alone, resulting in a 2.7 ml/min per 1.73 m 2 per year (95% CI, 0.1 to 5.3; P = 0.04) less decline with Chinese medicine. For UACR, the estimated proportion in the slope of change was 0.88 (95% CI, 0.75 to 1.02) and 0.99 (95% CI, 0.85 to 1.14) in participants treated with add-on Chinese medicine or standard care alone, respectively. The intergroup proportional difference (0.89, 11% slower increment in add-on Chinese medicine, 95% CI, 0.72 to 1.10; P = 0.28) did not reach statistical significance. Eighty-five adverse events were recorded from 50 participants (add-on Chinese medicine versus control: 22 [31%] versus 28 [36%]).
Conclusions: Rehmannia-6-based Chinese medicine treatment stabilized eGFR on top of standard care alone after 48 weeks in patients with type 2 diabetes, stage 2-3 CKD, and severely increased albuminuria.
Clinical trial registry: Semi-individualized Chinese Medicine Treatment as an Adjuvant Management for Diabetic Nephropathy (SCHEMATIC), NCT02488252 .
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
K.W. Chan reports employment with the University of Hong Kong and consultancy for Chinese Medicine Development Committee for Hong Kong Government and for Chinese Medicine Hospital Project Committee for Hong Kong Government. B.J. Cowling, Y. Feng, and W.H. Yiu report employment with The University of Hong Kong. T.P. Ip reports research funding from Amgen and Boehringer-Ingelheim. A.S.K. Kwong reports employment with Hospital Authority. S.L. Lui reports employment with Tung Wah Hospital; ownership interest in Hang Seng Bank (Hong Kong), Bank of China (Hong Kong), and Tracker Fund (Hong Kong); and research funding from Bayer Healthcare Pharmaceutical Inc. and Otsuka Pharmaceutical Development & Commercialization Inc. K.C.B. Tan reports employment with University of Hong Kong and speakers bureau for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, and Sanofi. S.C.W. Tang reports employment with the University of Hong Kong; consultancy for Eledon Pharmaceuticals and Travere Therapeutics, Inc.; honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Novartis; advisory or leadership roles as Theme and Subspecialties Editor of
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

