Rituximab vs Ocrelizumab in Relapsing-Remitting Multiple Sclerosis
- PMID: 37307006
- PMCID: PMC10262062
- DOI: 10.1001/jamaneurol.2023.1625
Rituximab vs Ocrelizumab in Relapsing-Remitting Multiple Sclerosis
Erratum in
-
Error in Figures.JAMA Neurol. 2023 Oct 1;80(10):1119. doi: 10.1001/jamaneurol.2023.2958. JAMA Neurol. 2023. PMID: 37603329 Free PMC article. No abstract available.
Abstract
Importance: Ocrelizumab, a humanized monoclonal antibody targeted against CD20+ B cells, reduces the frequency of relapses by 46% and disability worsening by 40% compared with interferon beta 1a in relapsing-remitting multiple sclerosis (MS). Rituximab, a chimeric monoclonal anti-CD20 agent, is often prescribed as an off-label alternative to ocrelizumab.
Objective: To evaluate whether the effectiveness of rituximab is noninferior to ocrelizumab in relapsing-remitting MS.
Design, setting, and participants: This was an observational cohort study conducted between January 2015 and March 2021. Patients were included in the treatment group for the duration of study therapy and were recruited from the MSBase registry and Danish MS Registry (DMSR). Included patients had a history of relapsing-remitting MS treated with ocrelizumab or rituximab, a minimum 6 months of follow-up, and sufficient data to calculate the propensity score. Patients with comparable baseline characteristics were 1:6 matched with propensity score on age, sex, MS duration, disability (Expanded Disability Status Scale), prior relapse rate, prior therapy, disease activity (relapses, disability accumulation, or both), magnetic resonance imaging lesion burden (missing values imputed), and country.
Exposure: Treatment with ocrelizumab or rituximab after 2015.
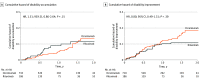
Main outcomes and measures: Noninferiority comparison of annualized rate of relapses (ARRs), with a prespecified noninferiority margin of 1.63 rate ratio. Secondary end points were relapse and 6-month confirmed disability accumulation in pairwise-censored groups.
Results: Of the 6027 patients with MS who were treated with ocrelizumab or rituximab, a total of 1613 (mean [SD] age; 42.0 [10.8] years; 1089 female [68%]) fulfilled the inclusion criteria and were included in the analysis (898 MSBase, 715 DMSR). A total of 710 patients treated with ocrelizumab (414 MSBase, 296 DMSR) were matched with 186 patients treated with rituximab (110 MSBase, 76 DMSR). Over a pairwise censored mean (SD) follow-up of 1.4 (0.7) years, the ARR ratio was higher in patients treated with rituximab than in those treated with ocrelizumab (rate ratio, 1.8; 95% CI, 1.4-2.4; ARR, 0.20 vs 0.09; P < .001). The cumulative hazard of relapses was higher among patients treated with rituximab than those treated with ocrelizumab (hazard ratio, 2.1; 95% CI, 1.5-3.0). No difference in the risk of disability accumulation was observed between groups. Results were confirmed in sensitivity analyses.
Conclusion: In this noninferiority comparative effectiveness observational cohort study, results did not show noninferiority of treatment with rituximab compared with ocrelizumab. As administered in everyday practice, rituximab was associated with a higher risk of relapses than ocrelizumab. The efficacy of rituximab and ocrelizumab administered at uniform doses and intervals is being further evaluated in randomized noninferiority clinical trials.
Conflict of interest statement
Figures



Comment in
-
New Approaches to Challenge Old Assumptions-B-Cell Depletion in Multiple Sclerosis.JAMA Neurol. 2023 Aug 1;80(8):775-777. doi: 10.1001/jamaneurol.2023.1079. JAMA Neurol. 2023. PMID: 37306978 No abstract available.
-
Comparative Efficacy of Disease-Modifying Treatments for Relapsing Multiple Sclerosis-Variations in Real-World Experience.JAMA Neurol. 2024 Jan 1;81(1):87. doi: 10.1001/jamaneurol.2023.4395. JAMA Neurol. 2024. PMID: 37983039 No abstract available.
References
-
- World Health Organization . WHO model list of essential medicines. Accessed October 11, 2022. https://www.who.int/medicines/publications/essentialmedicines/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

