Platelet functional abnormalities in pediatric patients with kaposiform hemangioendothelioma/Kasabach-Merritt phenomenon
- PMID: 37307200
- PMCID: PMC10463204
- DOI: 10.1182/bloodadvances.2022009590
Platelet functional abnormalities in pediatric patients with kaposiform hemangioendothelioma/Kasabach-Merritt phenomenon
Abstract
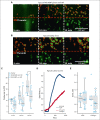
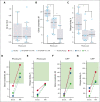
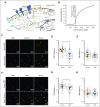
Kaposiform hemangioendothelioma (KHE) is a rare vascular tumor of infancy that is commonly associated with a life-threatening thrombocytopenic condition, Kasabach-Merritt phenomenon (KMP). Platelet CLEC-2, tumor podoplanin interaction is considered the key mechanism of platelet clearance in these patients. Here, we aimed to assess platelet functionality in such patients. Three groups of 6 to 9 children were enrolled: group A with KHE/KMP without hematologic response (HR) to therapy; group B with KHE/KMP with HR; and group C with healthy children. Platelet functionality was assessed by continuous and end point flow cytometry, low-angle light scattering analysis (LaSca), fluorescent microscopy of blood smears, and ex vivo thrombi formation. Platelet integrin activation in response to a combination of CRP (GPVI agonist) and TRAP-6 (PAR1 agonist), as well as calcium mobilization and integrin activation in response to CRP or rhodocytin (CLEC-2 agonist) alone, were significantly diminished in groups A and B. At the same time, platelet responses to ADP with or without TRAP-6 were unaltered. Thrombi formation from collagen in parallel plate flow chambers was also noticeably decreased in groups A and B. In silico analysis of these results predicted diminished amounts of CLEC-2 on the platelet surface of patients, which was further confirmed by immunofluorescence microscopy and flow cytometry. In addition, we also noted a decrease in GPVI levels on platelets from group A. In KHE/KMP, platelet responses induced by CLEC-2 or GPVI activation are impaired because of the diminished number of receptors on the platelet surface. This impairment correlates with the severity of the disease and resolves as the patient recovers.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Enjolras O, Wassef M, Mazoyer E, et al. Infants with Kasabach-Merritt syndrome do not have “true” hemangiomas. J Pediatr. 1997;130(4):631–640. - PubMed
-
- Kasabach H, Merritt K. Capillary hemangioma with extensive purpura. Am J Dis Child. 1940;59(5):1063–1070.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

