Retrospective characterization of nodal marginal zone lymphoma
- PMID: 37307213
- PMCID: PMC10469082
- DOI: 10.1182/bloodadvances.2022009587
Retrospective characterization of nodal marginal zone lymphoma
Abstract
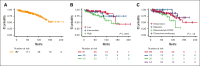
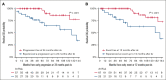
Nodal marginal zone lymphoma (NMZL) is a rare non-Hodgkin B-cell lymphoma that has historically been difficult to define, though is now formally recognized by the World Health Organization Classification. To better characterize the clinical outcomes of patients with NMZL, we reviewed a sequential cohort of 187 patients with NMZL to describe baseline characteristics, survival outcomes, and time-to-event data. Initial management strategies were classified into five categories: observation, radiation, anti-CD20 monoclonal antibody therapy, chemoimmunotherapy, or other. Baseline Follicular Lymphoma International Prognostic Index scores were calculated to evaluate prognosis. A total of 187 patients were analyzed. The five-year overall survival was 91% (95% confidence interval [CI], 87-95), with a median follow-up time of 71 months (range, 8-253) among survivors. A total of 139 patients received active treatment at any point, with a median follow-up time of 56 months (range, 13-253) among survivors who were never treated. The probability of remaining untreated at five years was 25% (95% CI, 19-33). For those initially observed, the median time to active treatment was 72 months (95% CI, 49-not reached). For those who received at least one active treatment, the cumulative incidence of receiving a second active treatment at 60 months was 37%. Transformation to large B-cell lymphoma was rare, with a cumulative incidence of 15% at 10 years. In summary, our series is a large cohort of uniformly diagnosed NMZL with detailed analyses of survival and time to event analyses. We showed that NMZL commonly presents as an indolent lymphoma for which initial observation is often a reasonable strategy.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests related to the presented work. Outside the scope of this work, R.S., E.D., M.O., Z.D.E.-P., P.A.H., D.J.S., A.M.I., and C.N.O. have no disclosures. A.D. served as a consultant for Incyte, EUSA Pharma, Loxo and receives research support from Roche and Takeda. B.S.I. has received honorarium from GT Medical Technologies, Inc. C.L.B. is currently employed by Genentech. P.C.C. has stock or stock options in Bristol Myers Squibb, Johnson and Johnson, Pfizer, AstraZeneca, GSK, Novartis. L.F. receives research funding and consultancy fees from Genmab, AbbVie and Roche/Genentech and has received honoraria/participated on advisory boards for ADCT, SeaGen and AstraZeneca. S.M.H. research support from ADC Therapeutics, Affimed, Aileron, Celgene, Crispr Therapeutics, Daiichi Sankyo, Forty Seven, Inc, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio, consultancy fees from Acrotech Biopharma, ADC Therapeutics, Astex, Auxilus Pharma, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, Vividion Therapeutics and Yingli Pharma Limited. N.K. receives research funding form SeaGen. O.B.L. served on advisory board for Morphosys, Inc. J.K.L. serves as a consult for TG therapeutics and Epizyme. M.J.M has received honoraria from Genentech, Roche, GlaxoSmithKline, Bayer, Pharmacyclics, Janssen, SeaGen, Immunovaccine Technologies, Takeda, and Epizyme, has participated in a consulting or advisory role for Genentech, Bayer, Merck, Juno Therapeutics, Roche, Teva, Rocket Medical, SeaGen, Daiichi Sankyo, Takeda, and Epizyme, and has received research funding from Genentech, Roche, GlaxoSmithKline, IGM Biosciences, Bayer, Pharmacyclis, Janssen, Rocket Medical, SeaGen, Immunovaccine Technologies. A.J.M. has research support from ADC Therapeutics, Beigene, Miragen, SeaGen, Merck, Bristol Myers Squibb, Incyte, and SecuraBio, honorarium from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd, Merck, Seattle Genetics, and Takeda. A.N. has grant/research support from Pharmacyclics/AbbVie; Kite/Gilead, Cornerstone, consultancy role from Janssen, Morphosys, Cornerstone, Epizyme, EUSA, TG therapeutics, ADC Therapeutics, and AstraZeneca. M.L.P. has advisory/consultancy roles with Novartis, Synthekine, BeiGene, Kite and MustangBio. S.V. has advisory/consultancy roles with Novartis, Synthekine, BeiGene, Kite MustangBio. A.D.Z. has received financial compensation for consulting from Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, Morphosys, AbbVie, AstraZeneca, MEI Pharma, research funding from MEI Pharmaceuticals, Genentech/Roche, BeiGene, NIH SPORE -PI (receives salary support and funds awarded to MSK) and has served on the data monitoring committee for BeiGene (Chair) and BMS/Celgene/Juno. G.S. has received financial compensations for participating on advisory boards or consulting from AbbVie, Bayer, BeiGene, BMS/Celgene, Epizyme, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo, Miltenyi, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, Regeneron and Takeda and is a shareholder in Owkin. A.K. has received research funding from AbbVie Pharmaceuticals, Adaptive Biotechnologies, Celgene, Pharmacyclics, Seattle Genetics, AstraZeneca, and Loxo Oncology/Lilly and has received financial compensation for participating on advisory board for Celgene, Genentech, Kite Pharmaceuticals, Loxo Oncology/Lilly, AstraZeneca.
The current affiliation for C.L.B. is Genentech, New York, NY.
Figures
References
-
- Piris MA, Rivas C, Morente M, Cruz MA, Rubio C, Oliva H. Monocytoid B-cell lymphoma, a tumour related to the marginal zone. Histopathology. 1988;12(4):383–392. - PubMed
-
- Nathwani BN, Drachenberg MR, Hernandez AM, Levine AM, Sheibani K. Nodal monocytoid B-cell lymphoma (nodal marginal-zone B-cell lymphoma) Semin Hematol. 1999;36(2):128–138. - PubMed
-
- Cousar JB, McGinn DL, Glick AD, List AF, Collins RD. Report of an unusual lymphoma arising from parafollicular B-lymphocytes (PBLs) or so-called monocytoid lymphocytes. Am J Clin Pathol. 1987;87(1):121–128. - PubMed