Spatial dynamics of malaria transmission
- PMID: 37307282
- PMCID: PMC10289676
- DOI: 10.1371/journal.pcbi.1010684
Spatial dynamics of malaria transmission
Abstract
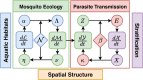
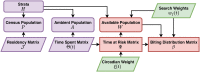
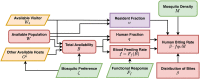
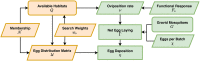
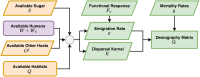
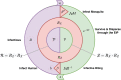
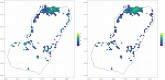
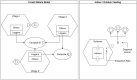
The Ross-Macdonald model has exerted enormous influence over the study of malaria transmission dynamics and control, but it lacked features to describe parasite dispersal, travel, and other important aspects of heterogeneous transmission. Here, we present a patch-based differential equation modeling framework that extends the Ross-Macdonald model with sufficient skill and complexity to support planning, monitoring and evaluation for Plasmodium falciparum malaria control. We designed a generic interface for building structured, spatial models of malaria transmission based on a new algorithm for mosquito blood feeding. We developed new algorithms to simulate adult mosquito demography, dispersal, and egg laying in response to resource availability. The core dynamical components describing mosquito ecology and malaria transmission were decomposed, redesigned and reassembled into a modular framework. Structural elements in the framework-human population strata, patches, and aquatic habitats-interact through a flexible design that facilitates construction of ensembles of models with scalable complexity to support robust analytics for malaria policy and adaptive malaria control. We propose updated definitions for the human biting rate and entomological inoculation rates. We present new formulas to describe parasite dispersal and spatial dynamics under steady state conditions, including the human biting rates, parasite dispersal, the "vectorial capacity matrix," a human transmitting capacity distribution matrix, and threshold conditions. An [Formula: see text] package that implements the framework, solves the differential equations, and computes spatial metrics for models developed in this framework has been developed. Development of the model and metrics have focused on malaria, but since the framework is modular, the same ideas and software can be applied to other mosquito-borne pathogen systems.
Copyright: © 2023 Wu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Levin S. The Problem of Relevant Detail. In: Busenberg S, Martelli M, editors. Differential Equations Models in Biology, Epidemiology and Ecology: Proceedings of a Conference held in Claremont California, January 13–16, 1990. Berlin, Heidelberg: Springer Berlin Heidelberg; 1991. p. 9–15.
-
- Radcliffe J. The initial geographical spread of host-vector and carrier-borne epidemics. J Appl Probab. 1973;10(4):703–717. doi: 10.2307/3212374 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

