Monitoring nutrients in plants with genetically encoded sensors: achievements and perspectives
- PMID: 37307576
- PMCID: PMC10469547
- DOI: 10.1093/plphys/kiad337
Monitoring nutrients in plants with genetically encoded sensors: achievements and perspectives
Abstract
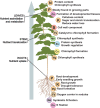
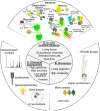
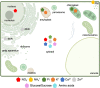
Understanding mechanisms of nutrient allocation in organisms requires precise knowledge of the spatiotemporal dynamics of small molecules in vivo. Genetically encoded sensors are powerful tools for studying nutrient distribution and dynamics, as they enable minimally invasive monitoring of nutrient steady-state levels in situ. Numerous types of genetically encoded sensors for nutrients have been designed and applied in mammalian cells and fungi. However, to date, their application for visualizing changing nutrient levels in planta remains limited. Systematic sensor-based approaches could provide the quantitative, kinetic information on tissue-specific, cellular, and subcellular distributions and dynamics of nutrients in situ that is needed for the development of theoretical nutrient flux models that form the basis for future crop engineering. Here, we review various approaches that can be used to measure nutrients in planta with an overview over conventional techniques, as well as genetically encoded sensors currently available for nutrient monitoring, and discuss their strengths and limitations. We provide a list of currently available sensors and summarize approaches for their application at the level of cellular compartments and organelles. When used in combination with bioassays on intact organisms and precise, yet destructive analytical methods, the spatiotemporal resolution of sensors offers the prospect of a holistic understanding of nutrient flux in plants.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures
References
-
- Akerboom J, Rivera JDV, Guilbe MMR, Malavé ECA, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER, et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009:284(10):6455–6464. 10.1074/jbc.M807657200 - DOI - PMC - PubMed
-
- Anderson LA, Palmer T, Price NC, Bornemann S, Boxer DH, Pau RN. Characterisation of the molybdenum-responsive ModE regulatory protein and its binding to the promoter region of the modABCD (molybdenum transport) operon of Escherichia coli. Eur J Biochem. 1997:246(1):119–126. 10.1111/j.1432-1033.1997.00119.x - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

