The inhibition of YAP Signaling Prevents Chronic Biliary Fibrosis in the Abcb4-/- Model by Modulation of Hepatic Stellate Cell and Bile Duct Epithelium Cell Pathophysiology
- PMID: 37307826
- PMCID: PMC10796084
- DOI: 10.14336/AD.2023.0602
The inhibition of YAP Signaling Prevents Chronic Biliary Fibrosis in the Abcb4-/- Model by Modulation of Hepatic Stellate Cell and Bile Duct Epithelium Cell Pathophysiology
Abstract
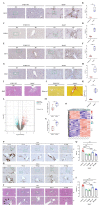
Primary sclerosing cholangitis (PSC) represents a chronic liver disease characterized by poor prognosis and lacking causal treatment options. Yes-associated protein (YAP) functions as a critical mediator of fibrogenesis; however, its therapeutic potential in chronic biliary diseases such as PSC remains unestablished. The objective of this study is to elucidate the possible significance of YAP inhibition in biliary fibrosis by examining the pathophysiology of hepatic stellate cells (HSC) and biliary epithelial cells (BEC). Human liver tissue samples from PSC patients were analyzed to assess the expression of YAP/connective tissue growth factor (CTGF) relative to non-fibrotic control samples. The pathophysiological relevance of YAP/CTGF in HSC and BEC was investigated in primary human HSC (phHSC), LX-2, H69, and TFK-1 cell lines through siRNA or pharmacological inhibition utilizing verteporfin (VP) and metformin (MF). The Abcb4-/- mouse model was employed to evaluate the protective effects of pharmacological YAP inhibition. Hanging droplet and 3D matrigel culture techniques were utilized to investigate YAP expression and activation status of phHSC under various physical conditions. YAP/CTGF upregulation was observed in PSC patients. Silencing YAP/CTGF led to inhibition of phHSC activation and reduced contractility of LX-2 cells, as well as suppression of epithelial-mesenchymal transition (EMT) in H69 cells and proliferation of TFK-1 cells. Pharmacological inhibition of YAP mitigated chronic liver fibrosis in vivo and diminished ductular reaction and EMT. YAP expression in phHSC was effectively modulated by altering extracellular stiffness, highlighting YAP's role as a mechanotransducer. In conclusion, YAP regulates the activation of HSC and EMT in BEC, thereby functioning as a checkpoint of fibrogenesis in chronic cholestasis. Both VP and MF demonstrate effectiveness as YAP inhibitors, capable of inhibiting biliary fibrosis. These findings suggest that VP and MF warrant further investigation as potential therapeutic options for the treatment of PSC.
Conflict of interest statement
FPR has received honoraria for lectures and/or travel support from Falk Foundation, Novartis and Ipsen, Gilead. NBK has received reimbursement of meeting attendance fees and travel expenses from EISAI and lecture honoraria from Falk. GD has received honoraria for lectures, teaching, advisory activities and travel support from AbbVie, Advanz/Intercept, Alexion, Falk Foundation, Gilead, Orphalan, Novartis, and Univar. AG has has received honoraria for lectures, teaching, advisory activities and travel support from AbbVie, Alexion, Bayer, BMS, CSL Behring, Eisai, Gilead, Intercept, Falk, Ipsen, MSD, Merz, Novartis, Pfizer, Roche, Sanofi-Aventis, Sequana and has received research support from Intercept und Falk (NAFLD CSG) and Novartis. EDT has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. All other authors declare that they have no conflict of interest.
Figures





References
-
- Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al.. (2015). The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol, 63:679-688. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
