Previous Dengue Infection among Children in Puerto Rico and Implications for Dengue Vaccine Implementation
- PMID: 37308104
- PMCID: PMC10397428
- DOI: 10.4269/ajtmh.23-0091
Previous Dengue Infection among Children in Puerto Rico and Implications for Dengue Vaccine Implementation
Abstract
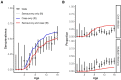
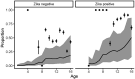
Limited dengue virus (DENV) seroprevalence estimates are available for Puerto Rico, which are needed to inform the potential use and cost-effectiveness of DENV vaccines. The Communities Organized to Prevent Arboviruses (COPA) is a cohort study initiated in 2018 in Ponce, Puerto Rico, to assess arboviral disease risk and provide a platform to evaluate interventions. We recruited participants from households in 38 study clusters, who were interviewed and provided a serum specimen. Specimens from 713 children aged 1 to 16 years during the first year of COPA were tested for the four DENV serotypes and ZIKV using a focus reduction neutralization assay. We assessed the seroprevalence of DENV and ZIKV by age and developed a catalytic model from seroprevalence and dengue surveillance data to estimate the force of infection for DENV during 2003-2018. Overall, 37% (n = 267) were seropositive for DENV; seroprevalence was 9% (11/128) among children aged 1 to 8 years and 44% (256/585) among children aged 9 to 16 years, exceeding the threshold over which DENV vaccination is deemed cost-effective. A total of 33% were seropositive for ZIKV, including 15% among children aged 0 to 8 years and 37% among children aged 9 to 16 years. The highest force of infection occurred in 2007, 2010, and 2012-2013, with low levels of transmission from 2016 to 2018. A higher proportion of children had evidence of multitypic DENV infection than expected, suggesting high heterogeneity in DENV risk in this setting.
Figures
Similar articles
-
Risk factors for infection with chikungunya and Zika viruses in southern Puerto Rico: A community-based cross-sectional seroprevalence survey.PLoS Negl Trop Dis. 2022 Jun 13;16(6):e0010416. doi: 10.1371/journal.pntd.0010416. eCollection 2022 Jun. PLoS Negl Trop Dis. 2022. PMID: 35696355 Free PMC article.
-
The seroepidemiology of dengue in a US military population based in Puerto Rico during the early phase of the Zika pandemic.PLoS Negl Trop Dis. 2022 Jan 21;16(1):e0009986. doi: 10.1371/journal.pntd.0009986. eCollection 2022 Jan. PLoS Negl Trop Dis. 2022. PMID: 35061659 Free PMC article.
-
Sentinel Enhanced Dengue Surveillance System - Puerto Rico, 2012-2022.MMWR Surveill Summ. 2024 May 30;73(3):1-29. doi: 10.15585/mmwr.ss7303a1. MMWR Surveill Summ. 2024. PMID: 38805389 Free PMC article.
-
Immune Responses to Dengue and Zika Viruses-Guidance for T Cell Vaccine Development.Int J Environ Res Public Health. 2018 Feb 23;15(2):385. doi: 10.3390/ijerph15020385. Int J Environ Res Public Health. 2018. PMID: 29473899 Free PMC article. Review.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
Cited by
-
Multiplex sample-sparing assay for detecting type-specific antibodies to Zika and dengue viruses: an assay development and validation study.Lancet Microbe. 2025 Feb;6(2):100951. doi: 10.1016/j.lanmic.2024.07.014. Epub 2024 Dec 25. Lancet Microbe. 2025. PMID: 39730005
-
Estimating the incidence of dengue in international air travelers from non-endemic countries between 2010-2019.PLoS Negl Trop Dis. 2025 Jul 9;19(7):e0013291. doi: 10.1371/journal.pntd.0013291. eCollection 2025 Jul. PLoS Negl Trop Dis. 2025. PMID: 40632801 Free PMC article.
-
Clinical Manifestations of Dengue in Children and Adults in a Hyperendemic Region of Colombia.Am J Trop Med Hyg. 2024 Mar 19;110(5):971-978. doi: 10.4269/ajtmh.23-0717. Print 2024 May 1. Am J Trop Med Hyg. 2024. PMID: 38507814 Free PMC article.
References
-
- Sridhar S. et al., 2018. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 379: 327–340. - PubMed
-
- Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Hj Muhammad Ismail H, Reynales H, Limkittikul K, Rivera-Medina DM, 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373: 1195–1206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

