Recurrent Circuits Amplify Corticofugal Signals and Drive Feedforward Inhibition in the Inferior Colliculus
- PMID: 37308295
- PMCID: PMC10401644
- DOI: 10.1523/JNEUROSCI.0626-23.2023
Recurrent Circuits Amplify Corticofugal Signals and Drive Feedforward Inhibition in the Inferior Colliculus
Abstract
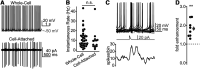
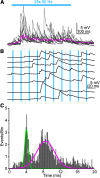
The inferior colliculus (IC) is a midbrain hub critical for perceiving complex sounds, such as speech. In addition to processing ascending inputs from most auditory brainstem nuclei, the IC receives descending inputs from auditory cortex that control IC neuron feature selectivity, plasticity, and certain forms of perceptual learning. Although corticofugal synapses primarily release the excitatory transmitter glutamate, many physiology studies show that auditory cortical activity has a net inhibitory effect on IC neuron spiking. Perplexingly, anatomy studies imply that corticofugal axons primarily target glutamatergic IC neurons while only sparsely innervating IC GABA neurons. Corticofugal inhibition of the IC may thus occur largely independently of feedforward activation of local GABA neurons. We shed light on this paradox using in vitro electrophysiology in acute IC slices from fluorescent reporter mice of either sex. Using optogenetic stimulation of corticofugal axons, we find that excitation evoked with single light flashes is indeed stronger in presumptive glutamatergic neurons compared with GABAergic neurons. However, many IC GABA neurons fire tonically at rest, such that sparse and weak excitation suffices to significantly increase their spike rates. Furthermore, a subset of glutamatergic IC neurons fire spikes during repetitive corticofugal activity, leading to polysynaptic excitation in IC GABA neurons owing to a dense intracollicular connectivity. Consequently, recurrent excitation amplifies corticofugal activity, drives spikes in IC GABA neurons, and generates substantial local inhibition in the IC. Thus, descending signals engage intracollicular inhibitory circuits despite apparent constraints of monosynaptic connectivity between auditory cortex and IC GABA neurons.SIGNIFICANCE STATEMENT Descending "corticofugal" projections are ubiquitous across mammalian sensory systems, and enable the neocortex to control subcortical activity in a predictive or feedback manner. Although corticofugal neurons are glutamatergic, neocortical activity often inhibits subcortical neuron spiking. How does an excitatory pathway generate inhibition? Here we study the corticofugal pathway from auditory cortex to inferior colliculus (IC), a midbrain hub important for complex sound perception. Surprisingly, cortico-collicular transmission was stronger onto IC glutamatergic compared with GABAergic neurons. However, corticofugal activity triggered spikes in IC glutamate neurons with local axons, thereby generating strong polysynaptic excitation and feedforward spiking of GABAergic neurons. Our results thus reveal a novel mechanism that recruits local inhibition despite limited monosynaptic convergence onto inhibitory networks.
Keywords: auditory; corticofugal; inferior colliculus; inhibition; synapse.
Copyright © 2023 the authors.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
