Deletion of Transferrin Receptor 1 in Parvalbumin Interneurons Induces a Hereditary Spastic Paraplegia-Like Phenotype
- PMID: 37308296
- PMCID: PMC10325000
- DOI: 10.1523/JNEUROSCI.2277-22.2023
Deletion of Transferrin Receptor 1 in Parvalbumin Interneurons Induces a Hereditary Spastic Paraplegia-Like Phenotype
Abstract
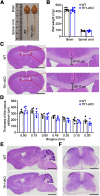
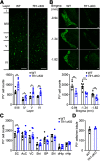
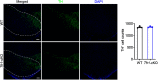
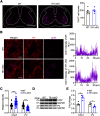
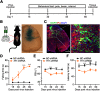
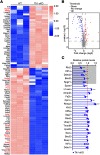
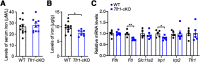
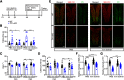
Hereditary spastic paraplegia (HSP) is a severe neurodegenerative movement disorder, the underlying pathophysiology of which remains poorly understood. Mounting evidence has suggested that iron homeostasis dysregulation can lead to motor function impairment. However, whether deficits in iron homeostasis are involved in the pathophysiology of HSP remains unknown. To address this knowledge gap, we focused on parvalbumin-positive (PV+) interneurons, a large category of inhibitory neurons in the central nervous system, which play a critical role in motor regulation. The PV+ interneuron-specific deletion of the gene encoding transferrin receptor 1 (TFR1), a key component of the neuronal iron uptake machinery, induced severe progressive motor deficits in both male and female mice. In addition, we observed skeletal muscle atrophy, axon degeneration in the spinal cord dorsal column, and alterations in the expression of HSP-related proteins in male mice with Tfr1 deletion in the PV+ interneurons. These phenotypes were highly consistent with the core clinical features of HSP cases. Furthermore, the effects on motor function induced by Tfr1 ablation in PV+ interneurons were mostly concentrated in the dorsal spinal cord; however, iron repletion partly rescued the motor defects and axon loss seen in both sexes of conditional Tfr1 mutant mice. Our study describes a new mouse model for mechanistic and therapeutic studies relating to HSP and provides novel insights into iron metabolism in spinal cord PV+ interneurons and its role in the regulation of motor functions.SIGNIFICANCE STATEMENT Iron is crucial for neuronal functioning. Mounting evidence suggests that iron homeostasis dysregulation can induce motor function deficits. Transferrin receptor 1 (TFR1) is thought to be the key component in neuronal iron uptake. We found that deletion of Tfr1 in parvalbumin-positive (PV+) interneurons in mice induced severe progressive motor deficits, skeletal muscle atrophy, axon degeneration in the spinal cord dorsal column, and alterations in the expression of hereditary spastic paraplegia (HSP)-related proteins. These phenotypes were highly consistent with the core clinical features of HSP cases and partly rescued by iron repletion. This study describes a new mouse model for the study of HSP and provides novel insights into iron metabolism in spinal cord PV+ interneurons.
Keywords: axon degeneration; hereditary spastic paraplegia; motor; parvalbumin; spinal cord; transferrin receptor 1.
Copyright © 2023 the authors.
Figures











Similar articles
-
Activating Striatal Parvalbumin Interneurons to Alleviate Chemotherapy-Induced Muscle Atrophy.J Cachexia Sarcopenia Muscle. 2025 Apr;16(2):e13782. doi: 10.1002/jcsm.13782. J Cachexia Sarcopenia Muscle. 2025. PMID: 40196908 Free PMC article.
-
Hereditary Spastic Paraplegia: Beyond Clinical Phenotypes toward a Unified Pattern of Central Nervous System Damage.Radiology. 2015 Jul;276(1):207-18. doi: 10.1148/radiol.14141715. Epub 2015 Jan 22. Radiology. 2015. PMID: 25611737
-
Chemogenetic Activation of Cortical Parvalbumin-Positive Interneurons Reverses Noise-Induced Impairments in Gap Detection.J Neurosci. 2021 Oct 20;41(42):8848-8857. doi: 10.1523/JNEUROSCI.2687-19.2021. Epub 2021 Aug 27. J Neurosci. 2021. PMID: 34452937 Free PMC article.
-
Ophthalmological changes in hereditary spastic paraplegia and other genetic diseases with spastic paraplegia.J Neurol Sci. 2020 Feb 15;409:116620. doi: 10.1016/j.jns.2019.116620. Epub 2019 Dec 6. J Neurol Sci. 2020. PMID: 31865189 Review.
-
Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms.Acta Neuropathol. 2013 Sep;126(3):307-28. doi: 10.1007/s00401-013-1115-8. Epub 2013 Jul 30. Acta Neuropathol. 2013. PMID: 23897027 Free PMC article. Review.
Cited by
-
Targeting MDM2 affects spastin protein levels and functions: implications for HSP treatment.Cell Death Discov. 2025 Feb 7;11(1):53. doi: 10.1038/s41420-025-02333-y. Cell Death Discov. 2025. PMID: 39920118 Free PMC article.
References
-
- Bastian TW, Prohaska JR, Georgieff MK, Anderson GW (2014) Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression. Endocrinology 155:1157–1167. 10.1210/en.2013-1571 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
