Mechanism of D-alanine transfer to teichoic acids shows how bacteria acylate cell envelope polymers
- PMID: 37308592
- PMCID: PMC10664464
- DOI: 10.1038/s41564-023-01411-0
Mechanism of D-alanine transfer to teichoic acids shows how bacteria acylate cell envelope polymers
Abstract
Bacterial cell envelope polymers are often modified with acyl esters that modulate physiology, enhance pathogenesis and provide antibiotic resistance. Here, using the D-alanylation of lipoteichoic acid (Dlt) pathway as a paradigm, we have identified a widespread strategy for how acylation of cell envelope polymers occurs. In this strategy, a membrane-bound O-acyltransferase (MBOAT) protein transfers an acyl group from an intracellular thioester onto the tyrosine of an extracytoplasmic C-terminal hexapeptide motif. This motif shuttles the acyl group to a serine on a separate transferase that moves the cargo to its destination. In the Dlt pathway, here studied in Staphylococcus aureus and Streptococcus thermophilus, the C-terminal 'acyl shuttle' motif that forms the crucial pathway intermediate is found on a transmembrane microprotein that holds the MBOAT protein and the other transferase together in a complex. In other systems, found in both Gram-negative and Gram-positive bacteria as well as some archaea, the motif is fused to the MBOAT protein, which interacts directly with the other transferase. The conserved chemistry uncovered here is widely used for acylation throughout the prokaryotic world.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures











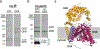

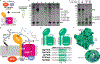
Similar articles
-
A partial reconstitution implicates DltD in catalyzing lipoteichoic acid d-alanylation.J Biol Chem. 2018 Nov 16;293(46):17985-17996. doi: 10.1074/jbc.RA118.004561. Epub 2018 Sep 20. J Biol Chem. 2018. PMID: 30237166 Free PMC article.
-
The mechanism of peptidoglycan O-acetylation in Gram-negative bacteria typifies bacterial MBOAT-SGNH acyltransferases.bioRxiv [Preprint]. 2024 Sep 19:2024.09.17.613324. doi: 10.1101/2024.09.17.613324. bioRxiv. 2024. Update in: J Biol Chem. 2025 Jun;301(6):108531. doi: 10.1016/j.jbc.2025.108531. PMID: 39345430 Free PMC article. Updated. Preprint.
-
Crystal structure of a membrane-bound O-acyltransferase.Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct 3. Nature. 2018. PMID: 30283133 Free PMC article.
-
Acyltransferases that Modify Cell Surface Polymers Across the Membrane.Biochemistry. 2025 Apr 15;64(8):1728-1749. doi: 10.1021/acs.biochem.4c00731. Epub 2025 Apr 2. Biochemistry. 2025. PMID: 40171682 Review.
-
A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria.Microbiol Mol Biol Rev. 2003 Dec;67(4):686-723. doi: 10.1128/MMBR.67.4.686-723.2003. Microbiol Mol Biol Rev. 2003. PMID: 14665680 Free PMC article. Review.
Cited by
-
Recent advances in polymeric nanoparticles for the treatment of hepatic diseases.Front Pharmacol. 2025 Jan 24;16:1528752. doi: 10.3389/fphar.2025.1528752. eCollection 2025. Front Pharmacol. 2025. PMID: 39925843 Free PMC article. Review.
-
Bacterial single-cell RNA sequencing captures biofilm transcriptional heterogeneity and differential responses to immune pressure.Nat Commun. 2024 Nov 24;15(1):10184. doi: 10.1038/s41467-024-54581-8. Nat Commun. 2024. PMID: 39580490 Free PMC article.
-
The mechanism of peptidoglycan O-acetylation in Gram-negative bacteria typifies bacterial MBOAT-SGNH acyltransferases.J Biol Chem. 2025 Jun;301(6):108531. doi: 10.1016/j.jbc.2025.108531. Epub 2025 Apr 23. J Biol Chem. 2025. PMID: 40280421 Free PMC article.
-
D-alanylation of lipoteichoic acids inhibitor provides anti-virulence and anti-resistance effects against methicillin-resistant Staphylococcus epidermidis.Antimicrob Agents Chemother. 2025 May 7;69(5):e0182224. doi: 10.1128/aac.01822-24. Epub 2025 Mar 21. Antimicrob Agents Chemother. 2025. PMID: 40116514 Free PMC article.
-
Structural insights into the transporting and catalyzing mechanism of DltB in LTA D-alanylation.Nat Commun. 2024 Apr 22;15(1):3404. doi: 10.1038/s41467-024-47783-7. Nat Commun. 2024. PMID: 38649359 Free PMC article.
References
-
- Percy MG & Gründling A Lipoteichoic acid synthesis and function in Gram-positive bacteria. Annu. Rev. Microbiol 68, 81–100 (2014). - PubMed
-
- Wecke J, Madela K & Fischer W The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143, 2953–2960 (1997). - PubMed
-
- Blackburn NT & Clarke AJ Characterization of soluble and membrane-bound family 3 lytic transglycosylases from Pseudomonas aeruginosa. Biochemistry 41, 1001–1013 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

