Itaconic acid underpins hepatocyte lipid metabolism in non-alcoholic fatty liver disease in male mice
- PMID: 37308721
- PMCID: PMC10290955
- DOI: 10.1038/s42255-023-00801-2
Itaconic acid underpins hepatocyte lipid metabolism in non-alcoholic fatty liver disease in male mice
Abstract
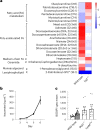
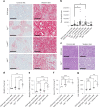
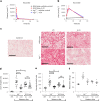
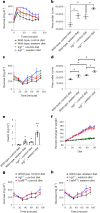
Itaconate, the product of the decarboxylation of cis-aconitate, regulates numerous biological processes. We and others have revealed itaconate as a regulator of fatty acid β-oxidation, generation of mitochondrial reactive oxygen species and the metabolic interplay between resident macrophages and tumors. In the present study, we show that itaconic acid is upregulated in human non-alcoholic steatohepatitis and a mouse model of non-alcoholic fatty liver disease. Male mice deficient in the gene responsible for itaconate production (immunoresponsive gene (Irg)-1) have exacerbated lipid accumulation in the liver, glucose and insulin intolerance and mesenteric fat deposition. Treatment of mice with the itaconate derivative, 4-octyl itaconate, reverses dyslipidemia associated with high-fat diet feeding. Mechanistically, itaconate treatment of primary hepatocytes reduces lipid accumulation and increases their oxidative phosphorylation in a manner dependent upon fatty acid oxidation. We propose a model whereby macrophage-derived itaconate acts in trans upon hepatocytes to modulate the liver's ability to metabolize fatty acids.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

