Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder
- PMID: 37308778
- PMCID: PMC10580367
- DOI: 10.1111/cns.14291
Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder
Abstract
Background: Neuronal abnormalities are closely associated with major depressive disorder (MDD). Available evidence suggests a role for microRNAs (miRNAs) in regulating the expression of genes involved in MDD. Hence, miRNAs that can be potential therapeutic targets need to be identified.
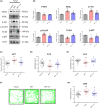
Methods: A mouse model of chronic unpredictable stress (CUS) was used to evaluate the function of miRNAs in MDD. miR-144-5p was screened from the hippocampi of CUS mice based on sequencing results. Adenovirus-associated vectors were used to overexpress or knockdown miR-144-5p in mice. BpV(pic) and LY294002 were used to determine the relationship between miR-144-5p target genes PTEN and TLR4 in neuronal impairment caused by miR-144-5p deficiency. Western blotting, immunofluorescence, ELISA immunosorbent assay, and Golgi staining were used to detect neuronal abnormalities. Serum samples from healthy individuals and patients with MDD were used to detect miR-144-5p levels in the serum and serum exosomes using qRT-PCR.
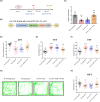
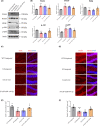
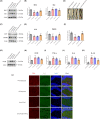
Results: miR-144-5p expression was significantly decreased within the hippocampal dentate gyrus (DG) of CUS mice. Upregulation of miR-144-5p in the DG ameliorated depression-like behavior in CUS mice and attenuated neuronal abnormalities by directly targeting PTEN and TLR4 expression. Furthermore, miR-144-5p knockdown in normal mice led to depression-like behavior via inducing neuronal abnormalities, including abnormal neurogenesis, neuronal apoptosis, altered synaptic plasticity, and neuroinflammation. miR-144-5p deficiency-mediated neuronal impairment was mediated by PI3K/Akt/FoxO1 signaling. Furthermore, miR-144-5p levels were downregulated in the sera of patients with MDD and associated with depressive symptoms. Consistently, serum exosome-derived miR-144-5p levels were decreased in patients with MDD.
Conclusion: miR-144-5p plays a vital role in regulating neuronal abnormalities in depression. Our findings provide translational evidence that miR-144-5p is a new potential therapeutic target for MDD.
Keywords: depression; miR-144-5p; neuroinflammation; neuronal destruction.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis.Neuropsychopharmacology. 2020 May;45(6):1050-1058. doi: 10.1038/s41386-020-0622-2. Epub 2020 Jan 27. Neuropsychopharmacology. 2020. PMID: 31986519 Free PMC article.
-
MicroRNA-204-5p reduction in rat hippocampus contributes to stress-induced pathology via targeting RGS12 signaling pathway.J Neuroinflammation. 2021 Oct 21;18(1):243. doi: 10.1186/s12974-021-02299-5. J Neuroinflammation. 2021. PMID: 34674723 Free PMC article.
-
LncRNA NPTN-IT1-201 Ameliorates Depressive-like Behavior by Targeting miR-142-5p and Regulating Inflammation and Apoptosis via BDNF.Curr Med Sci. 2024 Oct;44(5):971-986. doi: 10.1007/s11596-024-2917-8. Epub 2024 Aug 15. Curr Med Sci. 2024. PMID: 39145838
-
Synaptic plasticity and depression: the role of miRNAs dysregulation.Mol Biol Rep. 2022 Oct;49(10):9759-9765. doi: 10.1007/s11033-022-07461-7. Epub 2022 Apr 20. Mol Biol Rep. 2022. PMID: 35441941 Review.
-
A Systematic Review of Circulatory microRNAs in Major Depressive Disorder: Potential Biomarkers for Disease Prognosis.Int J Mol Sci. 2022 Jan 24;23(3):1294. doi: 10.3390/ijms23031294. Int J Mol Sci. 2022. PMID: 35163214 Free PMC article.
Cited by
-
Targeting miR-144-5p/ACSM1 Axis Alleviates Doxorubicin-Induced Heart Failure by Inhibiting Lipid Peroxidation.Curr Med Sci. 2025 May 5. doi: 10.1007/s11596-025-00053-z. Online ahead of print. Curr Med Sci. 2025. PMID: 40323471
-
Identifying the differentially expressed peripheral blood microRNAs in psychiatric disorders: a systematic review and meta-analysis.Front Psychiatry. 2024 May 17;15:1390366. doi: 10.3389/fpsyt.2024.1390366. eCollection 2024. Front Psychiatry. 2024. PMID: 38827444 Free PMC article.
-
Exosomal microRNAs in common mental disorders: Mechanisms, biomarker potential and therapeutic implications.World J Psychiatry. 2025 Aug 19;15(8):108933. doi: 10.5498/wjp.v15.i8.108933. eCollection 2025 Aug 19. World J Psychiatry. 2025. PMID: 40837802 Free PMC article. Review.
-
Effect and mechanism of miRNA-144-5p-regulated autophagy in older adults with Sarcopenia.Immun Ageing. 2025 Feb 14;22(1):7. doi: 10.1186/s12979-025-00499-8. Immun Ageing. 2025. PMID: 39953589 Free PMC article.
-
Clinical Insights into MicroRNAs in Depression: Bridging Molecular Discoveries and Therapeutic Potential.Int J Mol Sci. 2024 Mar 1;25(5):2866. doi: 10.3390/ijms25052866. Int J Mol Sci. 2024. PMID: 38474112 Free PMC article. Review.
References
-
- Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517. - PubMed
-
- Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32‐47. - PubMed
-
- Mozafari H, Amiri S, Mehr SE, et al. Minocycline attenuates depressive‐like behaviors in mice treated with the low dose of intracerebroventricular streptozotocin; the role of mitochondrial function and neuroinflammation. Mol Biol Rep. 2020;47(8):6143‐6153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

