Exosomal miR-125b-5p derived from adipose-derived mesenchymal stem cells enhance diabetic hindlimb ischemia repair via targeting alkaline ceramidase 2
- PMID: 37308908
- PMCID: PMC10259056
- DOI: 10.1186/s12951-023-01954-8
Exosomal miR-125b-5p derived from adipose-derived mesenchymal stem cells enhance diabetic hindlimb ischemia repair via targeting alkaline ceramidase 2
Abstract
Introduction: Ischemic diseases caused by diabetes continue to pose a major health challenge and effective treatments are in high demand. Mesenchymal stem cells (MSCs) derived exosomes have aroused broad attention as a cell-free treatment for ischemic diseases. However, the efficacy of exosomes from adipose-derived mesenchymal stem cells (ADSC-Exos) in treating diabetic lower limb ischemic injury remains unclear.
Methods: Exosomes were isolated from ADSCs culture supernatants by differential ultracentrifugation and their effect on C2C12 cells and HUVECs was assessed by EdU, Transwell, and in vitro tube formation assays separately. The recovery of limb function after ADSC-Exos treatment was evaluated by Laser-Doppler perfusion imaging, limb function score, and histological analysis. Subsequently, miRNA sequencing and rescue experiments were performed to figure out the responsible miRNA for the protective role of ADSC-Exos on diabetic hindlimb ischemic injury. Finally, the direct target of miRNA in C2C12 cells was confirmed by bioinformatic analysis and dual-luciferase report gene assay.
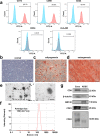
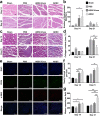
Results: ADSC-Exos have the potential to promote proliferation and migration of C2C12 cells and to promote HUVECs angiogenesis. In vivo experiments have shown that ADSC-Exos can protect ischemic skeletal muscle, promote the repair of muscle injury, and accelerate vascular regeneration. Combined with bioinformatics analysis, miR-125b-5p may be a key molecule in this process. Transfer of miR-125b-5p into C2C12 cells was able to promote cell proliferation and migration by suppressing ACER2 overexpression.
Conclusion: The findings revealed that miR-125b-5p derived from ADSC-Exos may play a critical role in ischemic muscle reparation by targeting ACER2. In conclusion, our study may provide new insights into the potential of ADSC-Exos as a treatment option for diabetic lower limb ischemia.
Keywords: Adipose-derived mesenchymal stem cells; Alkaline ceramidase 2; Bioinformatics analysis; Exosomes; Hindlimb ischemia; miR-125b-5p.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia.Stem Cell Res Ther. 2021 Jul 15;12(1):403. doi: 10.1186/s13287-021-02475-7. Stem Cell Res Ther. 2021. PMID: 34266474 Free PMC article.
-
Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury.Theranostics. 2021 Mar 11;11(11):5248-5266. doi: 10.7150/thno.54550. eCollection 2021. Theranostics. 2021. PMID: 33859745 Free PMC article.
-
Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome.Stem Cell Res Ther. 2022 Jul 26;13(1):349. doi: 10.1186/s13287-022-03037-1. Stem Cell Res Ther. 2022. PMID: 35883151 Free PMC article.
-
[Research advances on exosomes derived from adipose-derived mesenchymal stem cells in promoting diabetic wound healing].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 May 20;38(5):491-495. doi: 10.3760/cma.j.cn501120-20210218-00057. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35599426 Free PMC article. Review. Chinese.
-
Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models.Front Pharmacol. 2024 Oct 23;15:1481617. doi: 10.3389/fphar.2024.1481617. eCollection 2024. Front Pharmacol. 2024. PMID: 39508049 Free PMC article.
Cited by
-
Chinese herbal formula Huayu-Qiangshen-Tongbi decoction ameliorates rheumatoid arthritis through enhancing the release of exosomal miR-125b-5p derived from adipose-derived stem cells by CD63.Biol Res. 2025 Jul 10;58(1):47. doi: 10.1186/s40659-025-00628-z. Biol Res. 2025. PMID: 40640922 Free PMC article.
-
Exosome-based cell therapy for diabetic foot ulcers: Present and prospect.Heliyon. 2024 Oct 11;10(20):e39251. doi: 10.1016/j.heliyon.2024.e39251. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498056 Free PMC article. Review.
-
Exosomes from adipose-derived stem cells restore fibroblast function and accelerate diabetic wound healing.Heliyon. 2023 Nov 28;10(1):e22802. doi: 10.1016/j.heliyon.2023.e22802. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38163237 Free PMC article.
-
MicroRNAs in diabetic macroangiopathy.Cardiovasc Diabetol. 2024 Sep 16;23(1):344. doi: 10.1186/s12933-024-02405-w. Cardiovasc Diabetol. 2024. PMID: 39285459 Free PMC article. Review.
-
Therapeutic Applications of Stem Cell-Derived Exosomes.Int J Mol Sci. 2024 Mar 21;25(6):3562. doi: 10.3390/ijms25063562. Int J Mol Sci. 2024. PMID: 38542535 Free PMC article. Review.
References
-
- Babu M, Devi TD, Mäkinen P, Kaikkonen M, Lesch HP, Junttila S, et al. Differential promoter methylation of macrophage genes is associated with impaired vascular growth in ischemic muscles of hyperlipidemic and type 2 diabetic mice: genome-wide promoter methylation study. Circ Res. 2015;117:289–299. doi: 10.1161/CIRCRESAHA.115.306424. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

