Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer
- PMID: 37308954
- PMCID: PMC10262566
- DOI: 10.1186/s12967-023-04206-3
Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer
Abstract
Background: Non-small cell lung cancer (NSCLC) is a worldwide health threat with high annual morbidity and mortality. Chemotherapeutic drugs such as paclitaxel (PTX) have been widely applied clinically. However, systemic toxicity due to the non-specific circulation of PTX often leads to multi-organ damage, including to the liver and kidney. Thus, it is necessary to develop a novel strategy to enhance the targeted antitumor effects of PTX.
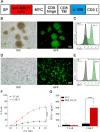
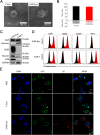
Methods: Here, we engineered exosomes derived from T cells expressing the chimeric antigen receptor (CAR-Exos), which targeted mesothelin (MSLN)-expressing Lewis lung cancer (MSLN-LLC) through the anti-MSLN single-chain variable fragment (scFv) of CAR-Exos. PTX was encapsulated into CAR-Exos (PTX@CAR-Exos) and administered via inhalation to an orthotopic lung cancer mouse model.
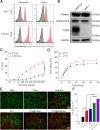
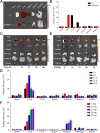
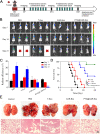
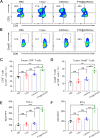
Results: Inhaled PTX@CAR-Exos accumulated within the tumor area, reduced tumor size, and prolonged survival with little toxicity. In addition, PTX@CAR-Exos reprogrammed the tumor microenvironment and reversed the immunosuppression, which was attributed to infiltrating CD8+ T cells and elevated IFN-γ and TNF-α levels.
Conclusions: Our study provides a nanovesicle-based delivery platform to promote the efficacy of chemotherapeutic drugs with fewer side effects. This novel strategy may ameliorate the present obstacles to the clinical treatment of lung cancer.
Keywords: CAR-T; Exosomes; Inhalation; Lung cancer; Paclitaxel; Targeted delivery.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Figures

Similar articles
-
Sequential Targeting Hybrid Nanovesicles Composed of Chimeric Antigen Receptor T-Cell-Derived Exosomes and Liposomes for Enhanced Cancer Immunochemotherapy.ACS Nano. 2023 Sep 12;17(17):16770-16786. doi: 10.1021/acsnano.3c03456. Epub 2023 Aug 25. ACS Nano. 2023. PMID: 37624742
-
Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation.Theranostics. 2019 Feb 28;9(6):1714-1727. doi: 10.7150/thno.30716. eCollection 2019. Theranostics. 2019. PMID: 31037133 Free PMC article.
-
Therapeutic effiacy of T cells expressing chimeric antigen receptor derived from a mesothelin-specific scFv in orthotopic human pancreatic cancer animal models.Neoplasia. 2022 Feb;24(2):98-108. doi: 10.1016/j.neo.2021.12.005. Epub 2021 Dec 23. Neoplasia. 2022. PMID: 34954452 Free PMC article.
-
Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives.Cancer Immunol Immunother. 2021 Mar;70(3):619-631. doi: 10.1007/s00262-020-02735-0. Epub 2020 Oct 6. Cancer Immunol Immunother. 2021. PMID: 33025047 Free PMC article. Review.
-
Mesothelin-targeted CAR-T cell therapy for solid tumors.Expert Opin Biol Ther. 2021 Apr;21(4):473-486. doi: 10.1080/14712598.2021.1843628. Epub 2020 Nov 12. Expert Opin Biol Ther. 2021. PMID: 33176519 Review.
Cited by
-
Focusing on exosomes to overcome the existing bottlenecks of CAR-T cell therapy.Inflamm Regen. 2024 Nov 4;44(1):45. doi: 10.1186/s41232-024-00358-x. Inflamm Regen. 2024. PMID: 39490997 Free PMC article. Review.
-
Natural killer cell-derived exosome-based cancer therapy: from biological roles to clinical significance and implications.Mol Cancer. 2024 Jun 29;23(1):134. doi: 10.1186/s12943-024-02045-4. Mol Cancer. 2024. PMID: 38951879 Free PMC article. Review.
-
Rab21-Targeted Nano Drug Delivery System-Based FFPG for Efficient Paclitaxel Delivery to Inhibit Lung Cancer Progression.Pharmaceutics. 2025 Jan 12;17(1):94. doi: 10.3390/pharmaceutics17010094. Pharmaceutics. 2025. PMID: 39861741 Free PMC article.
-
Exosomes in inflammation and cancer: from bench to bedside applications.Mol Biomed. 2025 Jun 10;6(1):41. doi: 10.1186/s43556-025-00280-9. Mol Biomed. 2025. PMID: 40490663 Free PMC article. Review.
-
Recent advances to address challenges in extracellular vesicle-based applications for lung cancer.Acta Pharm Sin B. 2024 Sep;14(9):3855-3875. doi: 10.1016/j.apsb.2024.06.010. Epub 2024 Jun 20. Acta Pharm Sin B. 2024. PMID: 39309489 Free PMC article. Review.
References
-
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

