The lncRNA HOTAIR: a pleiotropic regulator of epithelial cell plasticity
- PMID: 37308974
- PMCID: PMC10262438
- DOI: 10.1186/s13046-023-02725-x
The lncRNA HOTAIR: a pleiotropic regulator of epithelial cell plasticity
Abstract
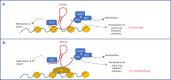
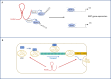
The epithelial-to-mesenchymal transition (EMT) is a trans-differentiation process that endows epithelial cells with mesenchymal properties, including motility and invasion capacity; therefore, its aberrant reactivation in cancerous cells represents a critical step to gain a metastatic phenotype. The EMT is a dynamic program of cell plasticity; many partial EMT states can be, indeed, encountered and the full inverse mesenchymal-to-epithelial transition (MET) appears fundamental to colonize distant secondary sites. The EMT/MET dynamics is granted by a fine modulation of gene expression in response to intrinsic and extrinsic signals. In this complex scenario, long non-coding RNAs (lncRNAs) emerged as critical players. This review specifically focuses on the lncRNA HOTAIR, as a master regulator of epithelial cell plasticity and EMT in tumors. Molecular mechanisms controlling its expression in differentiated as well as trans-differentiated epithelial cells are highlighted here. Moreover, current knowledge about HOTAIR pleiotropic functions in regulation of both gene expression and protein activities are described. Furthermore, the relevance of the specific HOTAIR targeting and the current challenges of exploiting this lncRNA for therapeutic approaches to counteract the EMT are discussed.
Keywords: Epithelial tumor progression; Epithelial-to-mesenchymal transition (EMT); HOTAIR; Long non-coding RNAs; Metastasis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

