Safety outcomes in patients with rheumatoid arthritis treated with abatacept: results from a multinational surveillance study across seven European registries
- PMID: 37308978
- PMCID: PMC10259009
- DOI: 10.1186/s13075-023-03067-x
Safety outcomes in patients with rheumatoid arthritis treated with abatacept: results from a multinational surveillance study across seven European registries
Abstract
Background: Patients with rheumatoid arthritis (RA) have an increased risk of infection and malignancy compared with the general population. Infection risk is increased further with the use of disease-modifying antirheumatic drugs (DMARDs), whereas evidence on whether the use of biologic DMARDs increases cancer risk remains equivocal. This single-arm, post-marketing study estimated the incidence of prespecified infection and malignancy outcomes in patients with RA treated with intravenous or subcutaneous abatacept.
Methods: Data were included from seven European RA quality registries: ATTRA (Anti-TNF Therapy in Rheumatoid Arthritis [Czech Republic]), DANBIO (Danish Rheumatologic Database), ROB-FIN (National Registry of Antirheumatic and Biological Treatment in Finland), ORA (Orencia and Rheumatoid Arthritis [France]), GISEA (Italian Group for the Study of Early Arthritis), BIOBADASER (Spanish Register of Adverse Events of Biological Therapies in Rheumatic Diseases), and the SCQM (Swiss Clinical Quality Management) system. Each registry is unique with respect to design, data collection, definition of the study cohort, reporting, and validation of outcomes. In general, registries defined the index date as the first day of abatacept treatment and reported data for infections requiring hospitalization and overall malignancies; data for other infection and malignancy outcomes were not available for every cohort. Abatacept exposure was measured in patient-years (p-y). Incidence rates (IRs) were calculated as the number of events per 1000 p-y of follow-up with 95% confidence intervals.
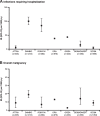
Results: Over 5000 patients with RA treated with abatacept were included. Most patients (78-85%) were female, and the mean age range was 52-58 years. Baseline characteristics were largely consistent across registries. Among patients treated with abatacept, IRs for infections requiring hospitalization across the registries ranged from 4 to 100 events per 1000 p-y, while IRs for overall malignancy ranged from 3 to 19 per 1000 p-y.
Conclusions: Despite heterogeneity between registries in terms of design, data collection, and ascertainment of safety outcomes, as well as the possibility of under-reporting of adverse events in observational studies, the safety profile of abatacept reported here was largely consistent with previous findings in patients with RA treated with abatacept, with no new or increased risks of infection or malignancy.
Keywords: Abatacept; Biologic DMARD; Infections; Malignancy; Rheumatoid arthritis; Safety.
© 2023. The Author(s).
Conflict of interest statement
AD: employee and shareholder: Bristol Myers Squibb (at the time of analysis); employee: Pfizer (current); MLH: grant/research support: AbbVie, Biogen, Bristol Myers Squibb, Merck, Novartis, Pfizer, Roche, Sandoz; consulting fees: Biogen, Celltrion, Eli Lilly, Merck, Orion Pharma, Pfizer, Samsung Bioepis; AF: grant/research support: AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Pfizer; speaker fees/honoraria: AB2Bio, AbbVie, Bristol Myers Squibb, Eli Lilly, Pfizer, Sandoz, Sanofi; J-EG: grant/research support, consulting fees: Bristol Myers Squibb, Eli Lilly, Pfizer; consulting fees: AbbVie, Bristol Myers Squibb, CSL Behring, Galapagos, Gilead, Merck Sharp & Dohme, Pfizer, Sanofi-Regeneron; FI: speaker fees/honoraria: AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, UCB; RC: speaker fees/honoraria: AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, UCB; TDK: employee: Bristol Myers Squibb (at the time of analysis), BeiGene USA, Inc. (current); DN: consulting fees, speaker fees/honoraria: AbbVie, Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, UCB; MVH, CS-P, FS-A: nothing to disclose; KP: speaker fees/honoraria: AbbVie, Amgen, Biogen, Bristol Myers Squibb, Egis, Merck Sharp & Dohme, Novartis, Pfizer, Roche, UCB; TCB: employee and shareholder: Bristol Myers Squibb; TAS: employee and shareholder: Bristol Myers Squibb (at the time of analysis); employee: Physicians Research Center (current); consulting fees: Bristol Myers Squibb.
Figures
References
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

