Model-based meta-analysis of ethnic differences and their variabilities in clearance of oral drugs classified by clearance mechanism
- PMID: 37309079
- PMCID: PMC10431045
- DOI: 10.1002/psp4.12980
Model-based meta-analysis of ethnic differences and their variabilities in clearance of oral drugs classified by clearance mechanism
Abstract
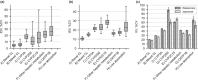
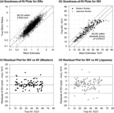
In this study, the ethnic ratios (ERs) of oral clearance between Japanese and Western populations were subjected to model-based meta-analysis (MBMA) for 81 drugs evaluated in 673 clinical studies. The drugs were classified into eight groups according to the clearance mechanism, and the ER for each group was inferred together with interindividual variability (IIV), interstudy variability (ISV), and inter-drug variability within a group (IDV) using the Markov chain Monte Carlo (MCMC) method. The ER, IIV, ISV, and IDV were dependent on the clearance mechanism, and, except for particular groups such as drugs metabolized by polymorphic enzymes or their clearance mechanism is not confirmative, the ethnic difference was found to be generally small. The IIV was well-matched across ethnicities, and the ISV was approximately half of the IIV as the coefficient of variation. To adequately assess ethnic differences in oral clearance without false detections, phase I studies should be designed with full consideration of the mechanism of clearance. This study suggests that the methodology of classifying drugs based on the mechanism that causes ethnic differences and performing MBMA with statistical techniques such as MCMC analysis is helpful for a rational understanding of ethnic differences and for strategic drug development.
© 2023 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

Similar articles
-
Inter-study variability in population pharmacokinetic meta-analysis: when and how to estimate it?J Pharm Sci. 2000 Feb;89(2):155-67. doi: 10.1002/(SICI)1520-6017(200002)89:2<155::AID-JPS3>3.0.CO;2-2. J Pharm Sci. 2000. PMID: 10688745 Review.
-
Use of Markov Chain Monte Carlo analysis with a physiologically-based pharmacokinetic model of methylmercury to estimate exposures in US women of childbearing age.Risk Anal. 2007 Aug;27(4):947-59. doi: 10.1111/j.1539-6924.2007.00934.x. Risk Anal. 2007. PMID: 17958503
-
Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance.Clin Pharmacokinet. 2011 Dec 1;50(12):809-22. doi: 10.2165/11594420-000000000-00000. Clin Pharmacokinet. 2011. PMID: 22087867
-
Importance of characterizing determinants of variability in exposure: application to dasatinib in subjects with chronic myeloid leukemia.J Clin Pharmacol. 2008 Nov;48(11):1254-69. doi: 10.1177/0091270008320604. Epub 2008 Sep 8. J Clin Pharmacol. 2008. PMID: 18779376
-
Inter-ethnic differences in pharmacokinetics-is there more that unites than divides?Pharmacol Res Perspect. 2021 Dec;9(6):e00890. doi: 10.1002/prp2.890. Pharmacol Res Perspect. 2021. PMID: 34725944 Free PMC article.
Cited by
-
New Horizon in Clinical Development Strategy in Japan Based on New Guideline on Japanese Phase 1 Studies Prior to Multi-Regional Clinical Trials.Clin Transl Sci. 2025 May;18(5):e70257. doi: 10.1111/cts.70257. Clin Transl Sci. 2025. PMID: 40395109 Free PMC article. No abstract available.
-
Utilising Endogenous Biomarkers in Drug Development to Streamline the Assessment of Drug-Drug Interactions Mediated by Renal Transporters: A Pharmaceutical Industry Perspective.Clin Pharmacokinet. 2024 Jun;63(6):735-749. doi: 10.1007/s40262-024-01385-0. Epub 2024 Jun 13. Clin Pharmacokinet. 2024. PMID: 38867094 Free PMC article. Review.
References
-
- Arnold FL, Kusama M, Ono S. Exploring differences in drug doses between Japan and Western countries. Clin Pharmacol Ther. 2010;87:714‐720. - PubMed
-
- Malinowski HJ, Westelinck A, Sato J, Ong T. Same drug, different dosing: differences in dosing for drugs approved in the United States, Europe, and Japan. J Clin Pharmacol. 2008;48:900‐908. - PubMed
-
- Ahsan CH, Renwick AG, Waller DG, Challenor VF, George CF, Amanullah M. The influence of dose and ethnic origins on the pharmacokinetics of nifedipine. Clin Pharmacol Ther. 1993;54:329‐338. - PubMed
-
- Caraco Y, Lagerstrom PO, Wood AJ. Ethnic and genetic determinants of omeprazole disposition and effect. Clin Pharmacol Ther. 1996;60:157‐167. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

