Long non-coding ribonucleic acid zinc finger E-box binding homeobox 1 antisense RNA 1 regulates myocardial fibrosis in diabetes through the Hippo-Yes-associated protein signaling pathway
- PMID: 37309277
- PMCID: PMC10360388
- DOI: 10.1111/jdi.13989
Long non-coding ribonucleic acid zinc finger E-box binding homeobox 1 antisense RNA 1 regulates myocardial fibrosis in diabetes through the Hippo-Yes-associated protein signaling pathway
Abstract
Aims/introduction: Fibrosis is the principle reason for heart failure in diabetes. Regarding the involvement of long non-coding ribonucleic acid zinc finger E-box binding homeobox 1 antisense 1 (ZEB1-AS1) in diabetic myocardial fibrosis, we explored its specific mechanism.
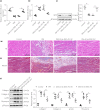
Materials and methods: Human cardiac fibroblasts (HCF) were treated with high glucose (HG) and manipulated with plasmid cloning deoxyribonucleic acid 3.1-ZEB1-AS1/microribonucleic acid (miR)-181c-5p mimic/short hairpin RNA specific to sirtuin 1 (sh-SIRT1). ZEB1-AS1, miR-181c-5p expression patterns, cell viability, collagen I and III, α-smooth muscle actin (α-SMA), fibronectin levels and cell migration were assessed by reverse transcription quantitative polymerase chain reaction, cell counting kit-8, western blot and scratch tests. Nuclear/cytosol fractionation assay verified ZEB1-AS1 subcellular localization. The binding sites between ZEB1-AS1 and miR-181c-5p, and between miR-181c-5p and SIRT1 were predicted and verified by Starbase and dual-luciferase assays. The binding of SIRT1 to Yes-associated protein (YAP) and YAP acetylation levels were detected by co-immunoprecipitation. Diabetic mouse models were established. SIRT1, collagen I, collagen III, α-SMA and fibronectin levels, mouse myocardium morphology and collagen deposition were determined by western blot, and hematoxylin-eosin and Masson trichrome staining.
Results: Zinc finger E-box binding homeobox 1 antisense 1 was repressed in HG-induced HCFs. ZEB1-AS1 overexpression inhibited HG-induced HCF excessive proliferation, migration and fibrosis, and diminished collagen I, collagen III, α-SMA and fibronectin protein levels in cells. miR-181c-5p had targeted binding sites with ZEB1-AS1 and SIRT1. SIRT1 silencing/miR-181c-5p overexpression abrogated ZEB1-AS1-inhibited HG-induced HCF proliferation, migration and fibrosis. ZEB1-AS1 suppressed HG-induced HCF fibrosis through SIRT1-mediated YAP deacetylation. ZEB1-AS1 and SIRT1 were repressed in diabetic mice, and miR-181c-5p was promoted. ZEB1-AS1 overexpression improved myocardial fibrosis in diabetic mice, and reduced collagen I, collagen III, α-SMA and fibronectin protein levels in myocardial tissues.
Conclusion: Long non-coding ribonucleic acid ZEB1-AS1 alleviated myocardial fibrosis through the miR-181c-5p-SIRT1-YAP axis in diabetic mice.
Keywords: Myocardial fibrosis; Yes-associated protein; Zinc finger E-box binding homeobox 1 antisense 1.
© 2023 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures






References
-
- Tao Z, Shi A, Zhao J. Epidemiological perspectives of diabetes. Cell Biochem Biophys 2015; 73: 181–185. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

