Gram-Scale Total Synthesis of (±)-Ibogamine
- PMID: 37310034
- PMCID: PMC12036526
- DOI: 10.1021/acs.orglett.3c01595
Gram-Scale Total Synthesis of (±)-Ibogamine
Abstract
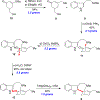
We describe the gram-scale total synthesis of (±)-ibogamine in nine steps and 24% overall yield. The approach features a Mitsunobu fragment coupling and macrocyclic Friedel-Crafts alkylation to establish the nitrogen-containing core of ibogamine. A regio- and diastereoselective hydroboration allows for simultaneous formation of the tetrahydroazepine and isoquinuclidine ring systems via sulfonamide deprotection and concomitant intramolecular cyclization.
Figures



Similar articles
-
Total Synthesis of Tabernanthine and Ibogaline: Rapid Access to Nosyl Tryptamines.European J Org Chem. 2024 Aug 12;27(30):e202400442. doi: 10.1002/ejoc.202400442. Epub 2024 Apr 26. European J Org Chem. 2024. PMID: 40896447 Free PMC article.
-
Diastereoselective Friedel-Crafts alkylation of hydronaphthalenes.J Org Chem. 2011 Nov 4;76(21):9031-45. doi: 10.1021/jo201781x. Epub 2011 Oct 5. J Org Chem. 2011. PMID: 21951196
-
Diastereoselective intramolecular Friedel-Crafts cyclizations of substituted methyl 2-(1H-indole-1-carbonyl)acrylates: efficient access to functionalized 1H-pyrrolo[1,2-a]indoles.Org Lett. 2011 Nov 4;13(21):5820-3. doi: 10.1021/ol202431x. Epub 2011 Oct 11. Org Lett. 2011. PMID: 21988209
-
Constructing Iboga alkaloids via C-H bond functionalization: examination of the direct and catalytic union of heteroarenes and isoquinuclidine alkenes.J Org Chem. 2015 Feb 20;80(4):2062-71. doi: 10.1021/jo5018102. Epub 2015 Jan 29. J Org Chem. 2015. PMID: 25633249
-
Enantioselective organocatalytic intramolecular ring-closing Friedel-Crafts-type alkylation of indoles.Org Lett. 2007 May 10;9(10):1847-50. doi: 10.1021/ol0703130. Epub 2007 Apr 12. Org Lett. 2007. PMID: 17428060
Cited by
-
Total Synthesis of Ervaoffine J and K.Chemistry. 2024 Mar 12;30(15):e202303985. doi: 10.1002/chem.202303985. Epub 2024 Jan 23. Chemistry. 2024. PMID: 38179797 Free PMC article.
-
Efficient and modular synthesis of ibogaine and related alkaloids.Nat Chem. 2025 Mar;17(3):412-420. doi: 10.1038/s41557-024-01714-7. Epub 2025 Feb 6. Nat Chem. 2025. PMID: 39915657 Free PMC article.
-
Total Synthesis of Tabernanthine and Ibogaline: Rapid Access to Nosyl Tryptamines.European J Org Chem. 2024 Aug 12;27(30):e202400442. doi: 10.1002/ejoc.202400442. Epub 2024 Apr 26. European J Org Chem. 2024. PMID: 40896447 Free PMC article.
-
Disrupting Substance Use Disorder: The Chemistry of Iboga Alkaloids.European J Org Chem. 2024 Sep 23;27(36):10.1002/ejoc.202400432. doi: 10.1002/ejoc.202400432. Epub 2024 Jul 7. European J Org Chem. 2024. PMID: 40895173 Free PMC article.
-
Discovery of Iboga-Derived Ligands for the Sigma‑2 Receptor.ACS Bio Med Chem Au. 2025 May 12;5(3):379-386. doi: 10.1021/acsbiomedchemau.5c00011. eCollection 2025 Jun 18. ACS Bio Med Chem Au. 2025. PMID: 40556780 Free PMC article.
References
-
- Lavaud C; Massiot G, The Iboga Alkaloids. Prog Chem Org Nat Prod 2017, 105, 89–136. - PubMed
-
- Augustine RL, Synthesis of iboga alkaloids and related compounds. Psychopharmacol Bull 1969, 5, 18. - PubMed
-
- Jana GK; Paul S; Sinha S, Progress in the Synthesis of Iboga-alkaloids and their Congeners. Org Prep Proced Int 2011, 43, 541–573.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

