Herpes Simplex Virus-1 ICP27 Nuclear Export Signal Mutants Exhibit Cell Type-Dependent Deficits in Replication and ICP4 Expression
- PMID: 37310267
- PMCID: PMC10373558
- DOI: 10.1128/jvi.01957-22
Herpes Simplex Virus-1 ICP27 Nuclear Export Signal Mutants Exhibit Cell Type-Dependent Deficits in Replication and ICP4 Expression
Abstract
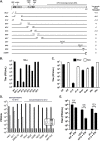
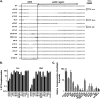
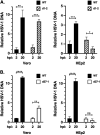
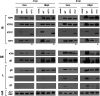
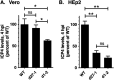
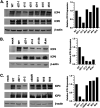
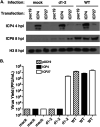
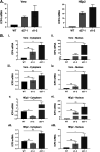
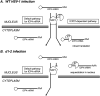
Herpes simplex virus type-1 (HSV-1) protein ICP27 is an essential immediate early (IE) protein that promotes the expression of viral early (E) and late (L) genes via multiple mechanisms. Our understanding of this complex regulatory protein has been greatly enhanced by the characterization of HSV-1 mutants bearing engineered alterations in the ICP27 gene. However, much of this analysis has been performed in interferon-deficient Vero monkey cells. Here, we assessed the replication of a panel of ICP27 mutants in several other cell types. Our analysis shows that mutants lacking ICP27's amino (N)-terminal nuclear export signal (NES) display a striking cell type-dependent growth phenotype, i.e., they grow semi-permissively in Vero and some other cells but are tightly blocked for replication in primary human fibroblasts and multiple human cell lines. This tight growth defect correlates with a failure of these mutants to replicate viral DNA. We also report that HSV-1 NES mutants are deficient in expressing the IE protein ICP4 at early times postinfection. Analysis of viral RNA levels suggests that this phenotype is due, at least in part, to a defect in the export of ICP4 mRNA to the cytoplasm. In combination, our results (i) show that ICP27's NES is critically important for HSV-1 replication in many human cells, and (ii) suggest that ICP27 plays a heretofore unappreciated role in the expression of ICP4. IMPORTANCE HSV-1 IE proteins drive productive HSV-1 replication. The major paradigm of IE gene induction, developed over many years, involves the parallel activation of the five IE genes by the viral tegument protein VP16, which recruits the host RNA polymerase II (RNAP II) to the IE gene promoters. Here, we provide evidence that ICP27 can enhance ICP4 expression early in infection. Because ICP4 is required for transcription of viral E and L genes, this finding may be relevant to understanding how HSV-1 enters and exits the latent state in neurons.
Keywords: HSV-1; ICP27; ICP4; molecular genetics; regulation of gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Roizman B, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601. In Knipe DM, Howley PM (ed), Fields virology, 5th ed, vol 2. Lippincott, Williams & Wilkins, Philadelphia, PA.
-
- Wald A, Corey L. 2007. Persistence in the population: epidemiology, transmission, chapter 36. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, UK. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

