Cost-Effectiveness Analysis of Increased Adalimumab Dose Intervals in Crohn's Disease Patients in Stable Remission: The Randomized Controlled LADI Trial
- PMID: 37310877
- PMCID: PMC10673815
- DOI: 10.1093/ecco-jcc/jjad101
Cost-Effectiveness Analysis of Increased Adalimumab Dose Intervals in Crohn's Disease Patients in Stable Remission: The Randomized Controlled LADI Trial
Abstract
Background and aims: We aimed to assess cost-effectiveness of increasing adalimumab dose intervals compared to the conventional dosing interval in patients with Crohn's disease [CD] in stable clinical and biochemical remission.
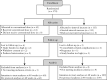
Design: We conducted a pragmatic, open-label, randomized controlled non-inferiority trial, comparing increased adalimumab intervals with the 2-weekly interval in adult CD patients in clinical remission. Quality of life was measured with the EQ-5D-5L. Costs were measured from a societal perspective. Results are shown as differences and incremental net monetary benefit [iNMB] at relevant willingness to accept [WTA] levels.
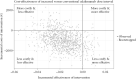
Results: We randomized 174 patients to the intervention [n = 113] and control [n = 61] groups. No difference was found in utility (difference: -0.017, 95% confidence interval [-0.044; 0.004]) and total costs (-€943, [-€2226; €1367]) over the 48-week study period between the two groups. Medication costs per patient were lower (-€2545, [-€2780; -€2192]) in the intervention group, but non-medication healthcare (+€474, [+€149; +€952]) and patient costs (+€365 [+€92; €1058]) were higher. Cost-utility analysis showed that the iNMB was €594 [-€2099; €2050], €69 [-€2908; €1965] and -€455 [-€4,096; €1984] at WTA levels of €20 000, €50 000 and €80 000, respectively. Increasing adalimumab dose intervals was more likely to be cost-effective at WTA levels below €53 960 per quality-adjusted life year. Above €53 960 continuing the conventional dose interval was more likely to be cost-effective.
Conclusion: When the loss of a quality-adjusted life year is valued at less than €53 960, increasing the adalimumab dose interval is a cost-effective strategy in CD patients in stable clinical and biochemical remission.
Clinical trial registration number: ClinicalTrials.gov, number NCT03172377.
Keywords: Adalimumab; Crohn’s disease; dose de-escalation.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
FMJ has received a research grant from ZonMW. RCAvL, RWMP, LJTS, WK, PJB, AGLB, IAMG, FHJW, MGVMR, PCJtB, JMJ, SVJ and ACIATLT have nothing to disclose. DJdJ has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Galapagos and held leadership roles in the Dutch Initiative on Crohn and Colitis and the IBD workgroup of the Dutch Gastroenterology Society. ACdV has received research grants from Takeda, Janssen, and Pfizer. RLW has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Ferring, Pfizer, Galapagos, AbbVie, and Janssen. TEHR payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie and has participated in the advisory board for Galapagos. MWMDL has received a grant for podcasts from Pfizer, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Jansen-Cilag and Galapagos, participated in advisory boards of BMS and Galapagos, and held leadership roles in the Elisabeth Twee Steden Ziekenhuis. AAvB has received research grants from Pfizer, Teva and ZonMW, consulting fees from Ferring, Galapagos, AbbVie and BMS, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Ferring, Galapagos and Janssen, support for attending meetings from Janssen, and held leadership roles in committees of the Dutch Gastroenterology Society and National Federation of Medical Specialists. BO has received research grants from Galapagos, Takeda, Ferring and Celltrion, has received consulting fees from AbbVie, Galapagos, Pfizer, Ferring, Takeda and Janssen, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda, Galapagos, AbbVie and Ferring and was chairman of the IBD committee of the Dutch Association of Gastroenterology [NVMDL]. MJP has received consulting fees from Takeda, Janssen, Galapagos and AbbVie and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen. NKdB has served as a speaker for AbbVie, Takeda and MSD. He has served as consultant and principal investigator for Takeda and TEVA. He has received research grants from Dr. Falk, Takeda, TEVA and MLDS. All outside the submitted work. RMH has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ITS and is a member of the national IBD committee of the Dutch Association for Gastroenterology and Hepatology. AEvdMdJ has received research grants from Galapagos, Nestlé, Cablon and Norgine, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Galapagos, Tramedico and Janssen-Cilag, and has participated in an advisory board for Ferring. FH has received funding for the present study from ZonMW, has received research grants from Janssen, AbbVie, Pfizer and Takeda, and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Janssen, Takeda and Pfizer. CJvdW has received funding for the present study from ZonMW, has received research grants from ZonMW, Falk and Pfizer, has received consulting fees from Janssen, Galapagos and Pfizer, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Ferring and AbbVie and had leadership roles in the European Crohn’s & Colitis organization, United European Gastroenterology council and the Dutch Association for Gastroenterology [NVGE].
Figures


References
-
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the charm trial. Gastroenterology 2007;132:52–65. - PubMed
-
- Brandse JF, Vos LM, Jansen J, et al. Serum concentration of anti-TNF antibodies, adverse effects and quality of life in patients with inflammatory bowel disease in remission on maintenance treatment. J Crohns Colitis 2015;9:973–81. - PubMed
-
- Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A.. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis 2018;24:742–51. - PubMed

