Impact of maternal SARS-CoV-2 booster vaccination on blood and breastmilk antibodies
- PMID: 37310982
- PMCID: PMC10263312
- DOI: 10.1371/journal.pone.0287103
Impact of maternal SARS-CoV-2 booster vaccination on blood and breastmilk antibodies
Abstract
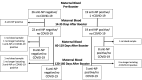
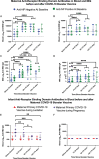
Maternal COVID-19 vaccination could protect infants who are ineligible for vaccine through antibody transfer during pregnancy and lactation. We measured the quantity and durability of SARS-CoV-2 antibodies in human milk and infant blood before and after maternal booster vaccination. Prospective cohort of lactating women immunized with primary and booster COVID-19 vaccines during pregnancy or lactation and their infants. Milk and blood samples from October 2021 to April 2022 were included. Anti-nucleoprotein (NP) and anti-receptor binding domain (RBD) IgG and IgA in maternal milk and maternal and infant blood were measured and compared longitudinally after maternal booster vaccine. Forty-five lactating women and their infants provided samples. 58% of women were anti-NP negative and 42% were positive on their first blood sample prior to booster vaccine. Anti-RBD IgG and IgA in milk remained significantly increased through 120-170 days after booster vaccine and did not differ by maternal NP status. Anti-RBD IgG and IgA did not increase in infant blood after maternal booster. Of infants born to women vaccinated in pregnancy, 74% still had positive serum anti-RBD IgG measured on average 5 months after delivery. Infant to maternal IgG ratio was highest for infants exposed to maternal primary vaccine during the second trimester compared to third trimester (0.85 versus 0.29; p<0.001). Maternal COVID-19 primary and booster vaccine resulted in robust and long-lasting transplacental and milk antibodies. These antibodies may provide important protection against SARS-CoV-2 during the first six months of life.
Copyright: © 2023 Rick et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: JVW serves on the Scientific Advisory Board for Quidel and a Data Safety Monitoring Board for GlaxoSmithKline, neither related to the present work. This does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures


References
-
- Center for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States 2022. [updated March 31, 2022April 4, 2022]. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-co....
-
- Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021;39(4):667–77. Epub 2020/12/22. doi: 10.1016/j.vaccine.2020.11.078 ; PubMed Central PMCID: PMC7833125. - DOI - PMC - PubMed
-
- Harwood R, Yan H, Talawila Da Camara N, Smith C, Ward J, Tudur-Smith C, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. EClinicalMedicine. 2022;44:101287. Epub 2022/02/17. doi: 10.1016/j.eclinm.2022.101287 ; PubMed Central PMCID: PMC8832134. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

