β-Cyclodextrins as affordable antivirals to treat coronavirus infection
- PMID: 37311279
- PMCID: PMC10247892
- DOI: 10.1016/j.biopha.2023.114997
β-Cyclodextrins as affordable antivirals to treat coronavirus infection
Abstract
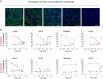
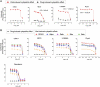
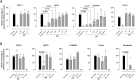
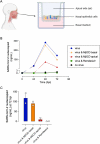
The SARS-CoV-2 pandemic made evident that there are only a few drugs against coronavirus. Here we aimed to identify a cost-effective antiviral with broad spectrum activity and high safety profile. Starting from a list of 116 drug candidates, we used molecular modelling tools to rank the 44 most promising inhibitors. Next, we tested their efficacy as antivirals against α and β coronaviruses, such as the HCoV-229E and SARS-CoV-2 variants. Four drugs, OSW-1, U18666A, hydroxypropyl-β-cyclodextrin (HβCD) and phytol, showed in vitro antiviral activity against HCoV-229E and SARS-CoV-2. The mechanism of action of these compounds was studied by transmission electron microscopy and by fusion assays measuring SARS-CoV-2 pseudoviral entry into target cells. Entry was inhibited by HβCD and U18666A, yet only HβCD inhibited SARS-CoV-2 replication in the pulmonary Calu-3 cells. Compared to the other cyclodextrins, β-cyclodextrins were the most potent inhibitors, which interfered with viral fusion via cholesterol depletion. β-cyclodextrins also prevented infection in a human nasal epithelium model ex vivo and had a prophylactic effect in the nasal epithelium of hamsters in vivo. All accumulated data point to β-cyclodextrins as promising broad-spectrum antivirals against different SARS-CoV-2 variants and distant alphacoronaviruses. Given the wide use of β-cyclodextrins for drug encapsulation and their high safety profile in humans, our results support their clinical testing as prophylactic antivirals.
Keywords: Antiviral; COVID-19; Coronavirus; Cyclodextrin; Drug repurposing; SARS-CoV-2; β-cyclodextrin.
Copyright © 2023 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CR, IFC, MS, RT, PO-G, AF-O, SF-S, NI-U, DP-Z, JM-B, DR-R, JPC-C, JAG and EN-D are inventors of a patent application for use of cyclodextrins to treat viral infection (PCT/EP2023/057735). The authors declare no other related competing interest.
Figures







References
-
- Agarwal A., Rochwerg B., Lamontagne F., Siemieniuk R.A.C., Agoritsas T., Askie L., Lytvyn L., Leo Y.-S., Macdonald H., Zeng L., Amin W., Barragan F.A.J., Bausch F.J., Burhan E., Calfee C.S., Cecconi M., Chanda D., Dat V.Q., De Sutter A., Du B., Geduld H., Gee P., Harley N., Hashmi M., Hunt B., Jehan F., Kabra S.K., Kanda S., Kim Y.-J., Kissoon N., Krishna S., Kuppalli K., Kwizera A., Lisboa T., Mahaka I., Manai H., Mino G., Nsutebu E., Preller J., Pshenichnaya N., Qadir N., Sabzwari S., Sarin R., Shankar-Hari M., Sharland M., Shen Y., Ranganathan S.S., Souza J.P., Stegemann M., Swanstrom R., Ugarte S., Venkatapuram S., Vuyiseka D., Wijewickrama A., Maguire B., Zeraatkar D., Bartoszko J.J., Ge L., Brignardello-Petersen R., Owen A., Guyatt G., Diaz J., Kawano-Dourado L., Jacobs M., Vandvik P.O. A living WHO guideline on drugs for covid-19. BMJ. 2020:m3379. doi: 10.1136/bmj.m3379. - DOI - PubMed
-
- Benet S., Blanch-Lombarte O., Ainsua-Enrich E., Pedreño-Lopez N., Muñoz-Basagoiti J., Raïch-Regué D., Perez-Zsolt D., Peña R., Jiménez E., de la Concepción M.L.R., Ávila C., Cedeño S., Escribà T., Romero-Martín L., Alarcón-Soto Y., Rodriguez-Lozano G.F., Miranda C., González S., Bailón L., Blanco J., Massanella M., Brander C., Clotet B., Paredes R., Esteve M., Izquierdo-Useros N., Carrillo J., Prado J.G., Moltó J., Mothe B. Limited humoral and specific T-cell responses after SARS-CoV-2 vaccination in PLWH with poor immune reconstitution. J. Infect. Dis. 2022 doi: 10.1093/infdis/jiac406. - DOI - PMC - PubMed
-
- Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S., Fagan P., Marvin J., Padilla D., Ravichandran V., Schneider B., Thanki N., Weissig H., Westbrook J.D., Zardecki C. The protein data bank. Acta Crystallogr. Sect. D. Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/S0907444902003451. - DOI - PubMed
-
- Bezerra B.B., Silva G.P.D., da, Coelho S.V.A., Correa I.A., Souza M.R.M., de, Macedo K.V.G., Matos B.M., Tanuri A., Matassoli F.L., Costa L.J., da, Hildreth J.E.K., Arruda L.B. de. Hydroxypropyl-beta-cyclodextrin (HP-BCD) inhibits SARS-CoV-2 replication and virus-induced inflammatory cytokines. Antivir. Res. 2022;205 doi: 10.1016/j.antiviral.2022.105373. - DOI - PMC - PubMed
-
- Bowers K.J., Chow E., Xu H., Dror R.O., Eastwood M.P., Gregersen B.A., Klepeis J.L., Istvan Kolossvary M., Moraes A., Sacerdoti F.D., Salmon J.K., Shan Y., Shaw D.E. Proceedings of the 2006 ACM/IEEE Conference on Supercomputing (SC ’06) Association for Computing Machinery; 2006. Scalable algorithms for molecular dynamics simulations on commodity clusters. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

