Metalloprotein enabled redox signal transduction in microbes
- PMID: 37311385
- PMCID: PMC10524656
- DOI: 10.1016/j.cbpa.2023.102331
Metalloprotein enabled redox signal transduction in microbes
Abstract
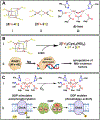
Microbes utilize numerous metal cofactor-containing proteins to recognize and respond to constantly fluctuating redox stresses in their environment. Gaining an understanding of how these metalloproteins sense redox events, and how they communicate such information downstream to DNA to modulate microbial metabolism, is a topic of great interest to both chemists and biologists. In this article, we review recently characterized examples of metalloprotein sensors, focusing on the coordination and oxidation state of the metals involved, how these metals are able to recognize redox stimuli, and how the signal is transmitted beyond the metal center. We discuss specific examples of iron, nickel, and manganese-based microbial sensors, and identify gaps in knowledge in the field of metalloprotein-based signal transduction pathways.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ambika Bhagi-Damodaran reports financial support was provided by Regents of the University of Minnesota. Ambika Bhagi-Damodaran reports financial support was provided by National Institutes of Health.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

