Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia
- PMID: 37311455
- PMCID: PMC11037504
- DOI: 10.1016/j.cmet.2023.05.008
Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia
Abstract
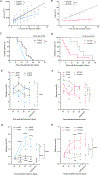
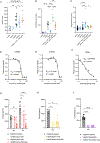
Glucose dependency of cancer cells can be targeted with a high-fat, low-carbohydrate ketogenic diet (KD). However, in IL-6-producing cancers, suppression of the hepatic ketogenic potential hinders the utilization of KD as energy for the organism. In IL-6-associated murine models of cancer cachexia, we describe delayed tumor growth but accelerated cachexia onset and shortened survival in mice fed KD. Mechanistically, this uncoupling is a consequence of the biochemical interaction of two NADPH-dependent pathways. Within the tumor, increased lipid peroxidation and, consequently, saturation of the glutathione (GSH) system lead to the ferroptotic death of cancer cells. Systemically, redox imbalance and NADPH depletion impair corticosterone biosynthesis. Administration of dexamethasone, a potent glucocorticoid, increases food intake, normalizes glucose levels and utilization of nutritional substrates, delays cachexia onset, and extends the survival of tumor-bearing mice fed KD while preserving reduced tumor growth. Our study emphasizes the need to investigate the effects of systemic interventions on both the tumor and the host to accurately assess therapeutic potential. These findings may be relevant to clinical research efforts that investigate nutritional interventions such as KD in patients with cancer.
Keywords: GDF-15; IL-6; NADPH; cachexia; cancer; corticosterone; ferroptosis; ketogenic diet; lipid peroxidation; steroid.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia.bioRxiv [Preprint]. 2023 Feb 18:2023.02.17.528937. doi: 10.1101/2023.02.17.528937. bioRxiv. 2023. Update in: Cell Metab. 2023 Jul 11;35(7):1147-1162.e7. doi: 10.1016/j.cmet.2023.05.008. PMID: 36824830 Free PMC article. Updated. Preprint.
Comment in
-
A Ketogenic Diet Induces Tumor Ferroptosis but Accelerates Cachexia.Cancer Discov. 2023 Aug 4;13(8):1758. doi: 10.1158/2159-8290.CD-RW2023-098. Cancer Discov. 2023. PMID: 37350638
-
Ketogenic diet in cancer: insufficient stress response?Nat Rev Endocrinol. 2023 Sep;19(9):497. doi: 10.1038/s41574-023-00869-6. Nat Rev Endocrinol. 2023. PMID: 37391433 No abstract available.
References
-
- Warburg O. (1925). The metabolism of carcinoma cells 1. J. Cancer Res. 9, 148–163. 10.1158/jcr.1925.148. - DOI
-
- Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, Voelker HU, Thiede A, and Coy JF (2008). Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 8, 1–12. 10.1186/1471-2407-8-122. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

