Parieto-occipital ERP indicators of gut mechanosensation in humans
- PMID: 37311748
- PMCID: PMC10264354
- DOI: 10.1038/s41467-023-39058-4
Parieto-occipital ERP indicators of gut mechanosensation in humans
Abstract
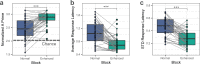
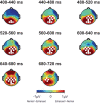
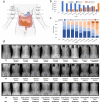
Understanding the neural processes governing the human gut-brain connection has been challenging due to the inaccessibility of the body's interior. Here, we investigated neural responses to gastrointestinal sensation using a minimally invasive mechanosensory probe by quantifying brain, stomach, and perceptual responses following the ingestion of a vibrating capsule. Participants successfully perceived capsule stimulation under two vibration conditions (normal and enhanced), as evidenced by above chance accuracy scores. Perceptual accuracy improved significantly during the enhanced relative to normal stimulation, which was associated with faster stimulation detection and reduced reaction time variability. Capsule stimulation induced late neural responses in parieto-occipital electrodes near the midline. Moreover, these 'gastric evoked potentials' showed intensity-dependent increases in amplitude and were significantly correlated with perceptual accuracy. Our results replicated in a separate experiment, and abdominal X-ray imaging localized most capsule stimulations to the gastroduodenal segments. Combined with our prior observation that a Bayesian model is capable of estimating computational parameters of gut-brain mechanosensation, these findings highlight a unique form of enterically-focused sensory monitoring within the human brain, with implications for understanding gut feelings and gut-brain interactions in healthy and clinical populations.
© 2023. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

