Temperature-triggered in situ forming lipid mesophase gel for local treatment of ulcerative colitis
- PMID: 37311749
- PMCID: PMC10264425
- DOI: 10.1038/s41467-023-39013-3
Temperature-triggered in situ forming lipid mesophase gel for local treatment of ulcerative colitis
Abstract
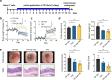
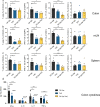
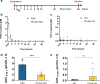
Ulcerative colitis is a chronic inflammatory bowel disease that strongly affects patient quality of life. Side effects of current therapies necessitate new treatment strategies that maximise the drug concentration at the site of inflammation, while minimizing systemic exposure. Capitalizing on the biocompatible and biodegradable structure of lipid mesophases, we present a temperature-triggered in situ forming lipid gel for topical treatment of colitis. We show that the gel is versatile and can host and release drugs of different polarities, including tofacitinib and tacrolimus, in a sustained manner. Further, we demonstrate its adherence to the colonic wall for at least 6 h, thus preventing leakage and improving drug bioavailability. Importantly, we find that loading known colitis treatment drugs into the temperature-triggered gel improves animal health in two mouse models of acute colitis. Overall, our temperature-triggered gel may prove beneficial in ameliorating colitis and decreasing adverse effects associated with systemic application of immunosuppressive treatments.
© 2023. The Author(s).
Conflict of interest statement
The authors M.C., M.R.S., R.A.G., R.M., K.H., A.M., P.K., P.L. and S.A. declare no competing interests. G.R. declares the following competing interests: consulting to Abbvie, Arena, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, Lilly, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; received speaker’s honoraria from Abbvie, Astra Zeneca, BMS, Celgene, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; received educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller. Gerhard Rogler is cofounder and head of the scientific advisory board of PharmaBiome. The TIF-Gel technology has been patented.
Figures



References
-
- Meier, J. Epidemiology. in Inflammatory Bowel Disease Nursing Manual (eds Sturm, A. & White, L.) 11–14 (Springer International Publishing, 2019).
-
- Manz, M., Michetti, P., Seibold, F., Rogler, G. & Beglinger, C. Treatment algorithm for moderate to severe ulcerative colitis. Swiss Medical Weekly141, 1–9 (2011). - PubMed

