Polymyxin B therapy based on therapeutic drug monitoring in carbapenem-resistant organisms sepsis: the PMB-CROS randomized clinical trial
- PMID: 37312218
- PMCID: PMC10262552
- DOI: 10.1186/s13054-023-04522-6
Polymyxin B therapy based on therapeutic drug monitoring in carbapenem-resistant organisms sepsis: the PMB-CROS randomized clinical trial
Abstract
Background: The appropriate administration regimen of polymyxin B is yet controversial. The present study aimed to explore the optimal dose of polymyxin B under therapeutic drug monitoring (TDM) guidance.
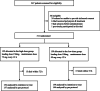
Methods: In China's Henan province, 26 hospitals participated in a randomized controlled trial. We included patients with sepsis caused by carbapenem-resistant Gram-negative bacteria (CR-GNB) susceptible to polymyxin B. The patients were randomly divided into a high-dose (HD) group or a low-dose (LD) group and received 150 mg loading dose, 75 mg every 12 h and 100 mg loading dose, 50 mg every 12 h, respectively. TDM was employed to determine if the dose of polymyxin B needs adjustment based on the area under the concentration-time curve across 24 h at a steady state (ssAUC0-24) of 50-100 mg h/L. The primary outcome was the 14-day clinical response, and the secondary outcomes included 28- and 14-day mortality.
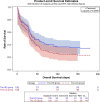
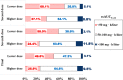
Results: This trial included 311 patients, with 152 assigned to the HD group and 159 assigned to the LD group. Intention-to-treat analysis showed that the 14-day clinical response was non-significant (p = 0.527): 95/152 (62.5%) in the HD group and 95/159 (59.7%) in the LD group. Kaplan-Meier's 180-day survival curve showed survival advantage in the HD group than in the LD group (p = 0.037). More patients achieved the target ssAUC0-24 in the HD than in the LD group (63.8% vs. 38.9%; p = 0.005) and in the septic shock subgroup compared to all subjects (HD group: 71.4% vs. 63.8%, p = 0.037; LD group: 58.3% vs. 38.9%, p = 0.0005). Also, the target AUC compliance was not correlated with clinical outcomes but with acute kidney injury (AKI) (p = 0.019). Adverse events did not differ between the HD and LD groups.
Conclusion: A fixed polymyxin B loading dose of 150 mg and a maintenance dose of 75 mg every 12 h was safe for patients with sepsis caused by CR-GNB and improves long-term survival. The increased AUC was associated with increased incidence of AKI, and TDM results were valued to prevent AKI. Trial registration Trial registration ClinicalTrials.gov: ChiCTR2100043208, Registration date: January 26, 2021.
Keywords: Carbapenem-resistant gram-negative bacteria; Optimization dose; Polymyxin B; Severe infection; Therapeutic drug monitoring.
© 2023. The Author(s).
Conflict of interest statement
None of the authors has any conflict of interest to declare.
Figures

Similar articles
-
Comprehensive analysis and novel insights into the efficacy of polymyxin B sulfate in the treatment of sepsis caused by carbapenem-resistant gram-negative bacteria.Am J Transl Res. 2024 Oct 15;16(10):6052-6063. doi: 10.62347/WBZU4331. eCollection 2024. Am J Transl Res. 2024. PMID: 39544731 Free PMC article.
-
Early appropriate therapy with polymyxin B reduces the mortality in burn sepsis caused by carbapenem-resistant gram-negative bacteria: a retrospective analysis.Eur J Clin Microbiol Infect Dis. 2025 Jun;44(6):1433-1442. doi: 10.1007/s10096-025-05119-3. Epub 2025 Apr 3. Eur J Clin Microbiol Infect Dis. 2025. PMID: 40178717
-
Efficacy and Safety Factors Related to Plasma Concentration-Optimized Polymyxin B Therapy in Treating Carbapenem-Resistant Gram-Negative Bacterial Infections in China.Infect Drug Resist. 2024 Jul 16;17:3057-3071. doi: 10.2147/IDR.S468890. eCollection 2024. Infect Drug Resist. 2024. PMID: 39050834 Free PMC article.
-
Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: is it superior to polymyxin monotherapy?Expert Rev Anti Infect Ther. 2023 Apr;21(4):387-429. doi: 10.1080/14787210.2023.2184346. Epub 2023 Mar 8. Expert Rev Anti Infect Ther. 2023. PMID: 36820511 Review.
-
Polymyxin B-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: a systematic review with meta-analysis and trial sequential analysis.Intensive Care Med. 2018 Feb;44(2):167-178. doi: 10.1007/s00134-017-5004-9. Epub 2017 Dec 4. Intensive Care Med. 2018. PMID: 29204670
Cited by
-
Current Therapeutic Approaches for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Infections.Antibiotics (Basel). 2024 Mar 15;13(3):261. doi: 10.3390/antibiotics13030261. Antibiotics (Basel). 2024. PMID: 38534696 Free PMC article. Review.
-
Efficiency of polymyxin B treatment against nosocomial infection: a systematic review and meta-analysis.Front Med (Lausanne). 2024 May 28;11:1400757. doi: 10.3389/fmed.2024.1400757. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38863886 Free PMC article.
-
Comprehensive analysis and novel insights into the efficacy of polymyxin B sulfate in the treatment of sepsis caused by carbapenem-resistant gram-negative bacteria.Am J Transl Res. 2024 Oct 15;16(10):6052-6063. doi: 10.62347/WBZU4331. eCollection 2024. Am J Transl Res. 2024. PMID: 39544731 Free PMC article.
-
Comparative pharmacokinetics of polymyxin B in critically ill elderly patients with extensively drug-resistant gram-negative bacteria infections.Front Pharmacol. 2024 Feb 1;15:1347130. doi: 10.3389/fphar.2024.1347130. eCollection 2024. Front Pharmacol. 2024. PMID: 38362145 Free PMC article.
-
Evaluation of polymyxin B AUC/MIC ratio for dose optimization in patients with carbapenem-resistant Klebsiella pneumoniae infection.Front Microbiol. 2023 Aug 22;14:1226981. doi: 10.3389/fmicb.2023.1226981. eCollection 2023. Front Microbiol. 2023. PMID: 37675417 Free PMC article.
References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/s1473-3099(17)30753-3. - DOI - PubMed
-
- Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28(4):521–547. doi: 10.1016/j.cmi.2021.11.025. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U2004110/the United Fund of National Natural Science Foundation of China
- 82172129/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
- Z20221343037/The central government guides local science and technology development funds
- SBGJ202101015)/Medical Science and Technology Tackling Plan Provincial and Ministerial Major Projects of Henan Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials

