Cell-Membrane-Coated and Cell-Penetrating Peptide-Conjugated Trimagnetic Nanoparticles for Targeted Magnetic Hyperthermia of Prostate Cancer Cells
- PMID: 37312240
- PMCID: PMC10316402
- DOI: 10.1021/acsami.3c07248
Cell-Membrane-Coated and Cell-Penetrating Peptide-Conjugated Trimagnetic Nanoparticles for Targeted Magnetic Hyperthermia of Prostate Cancer Cells
Abstract
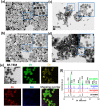
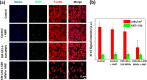
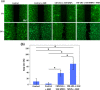
Prostate malignancy represents the second leading cause of cancer-specific death among the male population worldwide. Herein, enhanced intracellular magnetic fluid hyperthermia is applied in vitro to treat prostate cancer (PCa) cells with minimum invasiveness and toxicity and highly specific targeting. We designed and optimized novel shape-anisotropic magnetic core-shell-shell nanoparticles (i.e., trimagnetic nanoparticles - TMNPs) with significant magnetothermal conversion following an exchange coupling effect to an external alternating magnetic field (AMF). The functional properties of the best candidate in terms of heating efficiency (i.e., Fe3O4@Mn0.5Zn0.5Fe2O4@CoFe2O4) were exploited following surface decoration with PCa cell membranes (CM) and/or LN1 cell-penetrating peptide (CPP). We demonstrated that the combination of biomimetic dual CM-CPP targeting and AMF responsiveness significantly induces caspase 9-mediated apoptosis of PCa cells. Furthermore, a downregulation of the cell cycle progression markers and a decrease of the migration rate in surviving cells were observed in response to the TMNP-assisted magnetic hyperthermia, suggesting a reduction in cancer cell aggressiveness.
Keywords: cell membranes; cell-penetrating peptides; intracellular hyperthermia; prostate cancer; trimagnetic nanoparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Huo Y.; Yu J.; Gao S.. Synthesis and Biomedical applications of Magnetic Nanomaterials. In Magnetic-mediated Hyperthermia for Cancer Treatment: Research Progress and Clinical Trials; EDP Sciences: Les Ulis, 2022; pp 228–260.
-
- Li B.; Chen X.; Qiu W.; Zhao R.; Duan J.; Zhang S.; Pan Z.; Zhao S.; Guo Q.; Qi Y.; Wang W.; Deng L.; Ni S.; Sang Y.; Xue H.; Liu H.; Li G. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9 (17), 2105451.10.1002/advs.202105451. - DOI - PMC - PubMed
-
- Manohar A.; Vijayakanth V.; Vattikuti S. V. P.; Manivasagan P.; Jang E. S.; Chintagumpala K.; Kim K. H. Ca-Doped MgFe2O4 Nanoparticles for Magnetic Hyperthermia and Their Cytotoxicity in Normal and Cancer Cell Lines. ACS Appl. Nano Mater. 2022, 5 (4), 5847–5856. 10.1021/acsanm.2c01062. - DOI
-
- Kossatz S.; Grandke J.; Couleaud P.; Latorre A.; Aires A.; Crosbie-Staunton K.; Ludwig R.; Dähring H.; Ettelt V.; Lazaro-Carrillo A.; Calero M.; Sader M.; Courty J.; Volkov Y.; Prina-Mello A.; Villanueva A.; Somoza C.; Cortajarena A. L.; Miranda R.; Hilger I. Efficient Treatment of Breast Cancer Xenografts with Multifunctionalized Iron Oxide Nanoparticles Combining Magnetic Hyperthermia and Anti-Cancer Drug Delivery. Breast Cancer Res. 2015, 17 (1), 66.10.1186/s13058-015-0576-1. - DOI - PMC - PubMed
-
- Beola L.; Grazú V.; Fernández-Afonso Y.; Fratila R. M.; De Las Heras M.; De La Fuente J. M.; Gutiérrez L.; Asín L. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Appl. Mater. Interfaces 2021, 13 (11), 12982–12996. 10.1021/acsami.1c02338. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

