Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells
- PMID: 37312454
- PMCID: PMC10422001
- DOI: 10.1016/j.ymthe.2023.06.006
Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells
Abstract
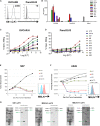
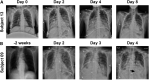
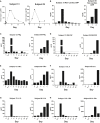
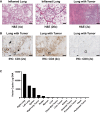
Multiple clinical studies have treated mesothelin (MSLN)-positive solid tumors by administering MSLN-directed chimeric antigen receptor (CAR) T cells. Although these products are generally safe, efficacy is limited. Therefore, we generated and characterized a potent, fully human anti-MSLN CAR. In a phase 1 dose-escalation study of patients with solid tumors, we observed two cases of severe pulmonary toxicity following intravenous infusion of this product in the high-dose cohort (1-3 × 108 T cells per m2). Both patients demonstrated progressive hypoxemia within 48 h of infusion with clinical and laboratory findings consistent with cytokine release syndrome. One patient ultimately progressed to grade 5 respiratory failure. An autopsy revealed acute lung injury, extensive T cell infiltration, and accumulation of CAR T cells in the lungs. RNA and protein detection techniques confirmed low levels of MSLN expression by benign pulmonary epithelial cells in affected lung and lung samples obtained from other inflammatory or fibrotic conditions, indicating that pulmonary pneumocyte and not pleural expression of mesothelin may lead to dose-limiting toxicity. We suggest patient enrollment criteria and dosing regimens of MSLN-directed therapies consider the possibility of dynamic expression of mesothelin in benign lung with a special concern for patients with underlying inflammatory or fibrotic conditions.
Keywords: MSLN; cancer; cell transfer therapy; chimeric antigen receptor (CAR) T cells; immunotherapy.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.H.J., R.M.Y., and M.M.D. are inventors on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receive license revenue from such licenses. J.L.B., B.E., K.M., and L.Z. are holders of stock options and patents with Novartis Institutes for Biomedical Research. R.M.Y. and M.M.D. are inventors on patents and/or patent applications licensed to Tmunity Therapeutics and receive license revenue from such licenses. C.H.J. and A.C. are scientific cofounders of Tmunity Therapeutics. C.H.J. is a scientific cofounder of Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune, Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Celldex, Decheng, Poseida, Verismo, and WIRB-Copernicus. B.E. and R.J.G. completed work on this study before becoming employees at Miltenyi Biotec and AstraZeneca, respectively. R.J.G. holds or may hold AstraZeneca stock. N.C.S. holds equity in Fate Therapeutics and Pfizer. J.A.F. has received grants and personal fees from Cartography Bio., grants from Tmunity Therapeutics, and personal fees from Retro Bio and Shennon Bio outside the submitted work. Additionally, J.A.F. holds patents related to CAR T cells for cancer that are licensed and associated with royalties. S.F.L. is an inventor on patents in the areas of CAR T and biomarkers at Penn that were assigned to Novartis; received research funding from Novartis, Tmunity, and Cabaletta; and consults for Kite/Gilead. M.M.D. is a consultant for Tmunity Therapeutics and is a Consultant and Member on the Scientific Advisory Board of Cellares Corporation.
Figures


References
-
- Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

