BPB1 regulates rice (Oryza sative L.) panicle length and panicle branch development by promoting lignin and inhibiting cellulose accumulation
- PMID: 37312745
- PMCID: PMC10248638
- DOI: 10.1007/s11032-023-01389-x
BPB1 regulates rice (Oryza sative L.) panicle length and panicle branch development by promoting lignin and inhibiting cellulose accumulation
Abstract
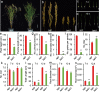
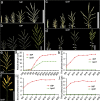
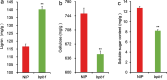
Panicle structure is one of the most important agronomic traits directly related to rice yield. This study identified a rice mutant basal primary branch 1 (bpb1), which exhibited a phenotype of reduced panicle length and arrested basal primary branch development. In addition, lignin content was found to be increased while cellulose content was decreased in bpb1 young panicles. Map-based cloning methods characterized the gene BPB1, which encodes a peptide transporter (PTR) family transporter. Phylogenetic tree analysis showed that the BPB1 family is highly conserved in plants, especially the PTR2 domain. It is worth noting that BPB1 is divided into two categories based on monocotyledonous and dicotyledonous plants. Transcriptome analysis showed that BPB1 mutation can promote lignin synthesis and inhibit cellulose synthesis, starch and sucrose metabolism, cell cycle, expression of various plant hormones, and some star genes, thereby inhibiting rice panicle length, resulting in basal primary branch development stagnant phenotypes. In this study, BPB1 provides new insights into the molecular mechanism of rice panicle structure regulation by BPB1 by regulating lignin and cellulose content and several transcriptional metabolic pathways.
Supplementary information: The online version contains supplementary material available at 10.1007/s11032-023-01389-x.
Keywords: Cellulose; Lignin; Primary branch development arrest; Short panicle.
© The Author(s), under exclusive licence to Springer Nature B.V. 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




References
-
- Ahmad I, X-p MENG, Kamran M, Shahzad A, Ahmad S, T-n LIU, Tie C, Q-f HAN. Effects of uniconazole with or without micronutrient on the lignin biosynthesis, lodging resistance, and winter wheat production in semiarid regions. J Integr Agric. 2020;19(1):62–77. doi: 10.1016/s2095-3119(19)62632-8. - DOI
LinkOut - more resources
Full Text Sources

