Design and implementation of a digital site-less clinical study of serial rapid antigen testing to identify asymptomatic SARS-CoV-2 infection
- PMID: 37313378
- PMCID: PMC10260333
- DOI: 10.1017/cts.2023.540
Design and implementation of a digital site-less clinical study of serial rapid antigen testing to identify asymptomatic SARS-CoV-2 infection
Abstract
Background: Rapid antigen detection tests (Ag-RDT) for SARS-CoV-2 with emergency use authorization generally include a condition of authorization to evaluate the test's performance in asymptomatic individuals when used serially. We aim to describe a novel study design that was used to generate regulatory-quality data to evaluate the serial use of Ag-RDT in detecting SARS-CoV-2 virus among asymptomatic individuals.
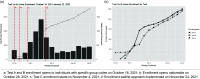
Methods: This prospective cohort study used a siteless, digital approach to assess longitudinal performance of Ag-RDT. Individuals over 2 years old from across the USA with no reported COVID-19 symptoms in the 14 days prior to study enrollment were eligible to enroll in this study. Participants throughout the mainland USA were enrolled through a digital platform between October 18, 2021 and February 15, 2022. Participants were asked to test using Ag-RDT and molecular comparators every 48 hours for 15 days. Enrollment demographics, geographic distribution, and SARS-CoV-2 infection rates are reported.
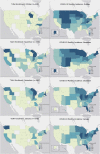
Key results: A total of 7361 participants enrolled in the study, and 492 participants tested positive for SARS-CoV-2, including 154 who were asymptomatic and tested negative to start the study. This exceeded the initial enrollment goals of 60 positive participants. We enrolled participants from 44 US states, and geographic distribution of participants shifted in accordance with the changing COVID-19 prevalence nationwide.
Conclusions: The digital site-less approach employed in the "Test Us At Home" study enabled rapid, efficient, and rigorous evaluation of rapid diagnostics for COVID-19 and can be adapted across research disciplines to optimize study enrollment and accessibility.
Keywords: Covid-19; digital trial; point-of-care diagnostics; rapid antigen tests; study recruitment.
© The Author(s) 2023.
Conflict of interest statement
VK is principal, and TS, SS, CN, ES, and EH are employees of the health care technology company CareEvolution, which was contracted to configure the smartphone study app, provide operational and logistical support, and collaborate on overall research approach. LS and LR are employees of Quest Diagnostics LLC, which was contracted to provide direct-to-consumer kits, logistical support for nationwide RT-PCR testing, and operational support for producing molecular testing results. DDM reports consulting and research grants from Bristol-Myers Squibb and Pfizer, consulting and research support from Fitbit, consulting, and research support from Flexcon, research grant from Boehringer Ingelheim, consulting from Avania, non-financial research support from Apple Computer, consulting/other support from Heart Rhythm Society. YCM has received tests from Quanterix, Becton-Dickinson, Ceres, and Hologic for research-related purposes, consults for Abbott on subjects unrelated to SARS-CoV-2, and receives funding support to Johns Hopkins University from miDiagnostics. LG is on a scientific advisory board for Moderna on projects unrelated to SARS-CoV-2. AS receives non-financial support from CareEvolution for collaborative research activities. Additional authors declare no financial or nonfinancial competing interests.
Figures
Update of
-
Finding a Needle in a Haystack: Design and Implementation of a Digital Site-less Clinical Study of Serial Rapid Antigen Testing to Identify Asymptomatic SARS-CoV-2 Infection.medRxiv [Preprint]. 2023 Jan 23:2022.08.04.22278274. doi: 10.1101/2022.08.04.22278274. medRxiv. 2023. Update in: J Clin Transl Sci. 2023 May 10;7(1):e120. doi: 10.1017/cts.2023.540. PMID: 35982663 Free PMC article. Updated. Preprint.
References
-
- Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised) | FDA. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed July 4, 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous