BMP6 knockdown enhances cardiac fibrosis in a mouse myocardial infarction model by upregulating AP-1/CEMIP expression
- PMID: 37313693
- PMCID: PMC10265437
- DOI: 10.1002/ctm2.1296
BMP6 knockdown enhances cardiac fibrosis in a mouse myocardial infarction model by upregulating AP-1/CEMIP expression
Abstract
Background: The cardiac repair process following a myocardial infarction is a key factor in patient prognosis. In this repair process, cardiac fibrosis takes a critically important role. Among those featured genes for fibrosis, transforming growth factor beta (TGF-β) is known to be involved in the fibrosis in various organs. And bone morphogenetic protein (BMP)6 belongs to the TGF-β superfamily. Although BMPs are known to play exclusive roles in cardiac repair processes, the character of BMP6 in cardiac remodelling remains unclear.
Purpose: This study aimed to investigate how BMP6 functioned in cardiac fibrosis following myocardial infarction (MI).
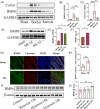
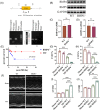
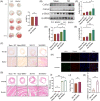
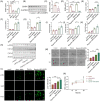
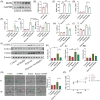
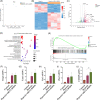
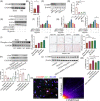
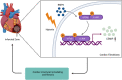
Results: In this paper, we demonstrated that BMP6 expression was upregulated after myocardial infarction in wild-type (WT) mice. Furthermore, BMP6-/- mice showed a more significant decline in cardiac function and lower survival curves after MI. An enlarged infarct area, increased fibrosis and more pronounced inflammatory infiltration were observed in BMP6-/- mice compared to WT mice. The expression of collagen I, collagen III and α-SMA was increased in BMP6-/- mice. In vitro, through gain-of-function and loss-of-function experiments, it was demonstrated that BMP6 decreases collagen secretion in fibroblasts. Mechanistically, knocking down BMP6 promoted AP-1 phosphorylation, which in turn promotes CEMIP expression, led to an acceleration in the progression of cardiac fibrosis. Finally, it was found that rhBMP6 would alleviate ventricular remodelling abnormalities after myocardial infarction.
Conclusion: Therefore, BMP6 may be a novel molecular target for improving myocardial fibrosis and cardiac function after myocardial infarction.
Keywords: bone morphogenetic protein 6; cardiac fibrosis; myocardial infarction.
© 2023 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

References
-
- Puymirat E, Simon T, Cayla G, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6‐month outcomes over a period of 20 years in the FAST‐MI program (French Registry of acute ST‐elevation or non‐ST‐elevation myocardial infarction) 1995 to 2015. Circulation. 2017;136(20):1908‐1919. - PubMed
-
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155‐2165. - PubMed
-
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233‐241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
